PHARMACOECONOMIC ANALISIS OF
DRUG PROVISION OF PATIENTS WITH BRONCHIAL ASTHMA
The
Tashkent pharmaceutical institute, The National university of Uzbekistan after
Mirzo Ulugbek
Republic
of Uzbekistan
N.D. Suyunov, Sh.F. Madrakhimov,
G.M.Ikramova,
SUMMARY
Questioning
was performed in the pharmaceutical market of Uzbekistan among 102 leading
physicians of specialized institutions on 91 medications (indicating their form
and dosage) used for the treatment of bronchial asthma. The aim of the study
was to perform VEN analysis, that is, determination of vital – V, essential – E
and non-essential – N preparations. Questioning data was treated by an
intellectual method. After distribution of medications into VEN groups,
preparations were revealed that should be more widely used in the treatment of
bronchial asthma in future. Most important groups of medications were
determined for the treatment of bronchial asthma in in-patient and out-patients
conditions.
Keywords: bronchial asthma, medications, expert
estimation, ABC and VEN-analysis, questioning, general estimation, intellectual
data processing.
Bronchial
asthma is one of the widespread diseases in the world. Epidemiological studies
conducted during the past few years have revealed that 4% to 10% of the world
population suffers from this disease. In Uzbekistan there has been a tendency
toward the growth of bronchial asthma rate. In the last 5 years the number of
patients with severe clinical forms of bronchial asthma increased from 0.7% to
17.5%, that is, almost in 20 times. At present, the number of asthmatic
patients accounts for 42% to 65% of all the patients with allergic diseases
applying to the Republican Specialized Allergy Research Center [5].
Economic
analysis of pharmacotherapy (pharmacoeconomics) represents a possible variant
to assess the efficacy of budget disbursement for the organization and
implementation of treatment process Studies on minimization, efficacy, utility
of expenses; comparison of expenses and results are the most used approaches to
the assessment of the pharmacoeconomic cost in healthcare and allow study
results to be directed at decision making in selection of drugs [6].
Pharmacoeconomic
ABC-, VEN- and frequency analyses are auxiliary types of the clinical-economic
analysis. Based on drug consumption parameters were divided into groups.
ABC-analysis – distribution of drugs to 3 groups according to expenses for each
of them in a total cost structure, from most to least. The groups A, B and C
include drugs accounting for 80%, 15% and 5% of expenses, respectively. The
group A usually accounts for 10-15%, group B for up to 50%, group C for up to
40% of all drugs under study. This method is used to determine priorities and
utility of drug expenses based on the
retrospective assessment of their real cost [3, 7].
To conduct
the study, permission was given by the Research Coordination Department of the
Ministry of Public Health of Uzbekistan. A questionnaire was worked out for
VEN-analysis to determine priorities of selection and purchase of drugs
according to their classification into vital and improving life quality (V),
essential (E) and non-essential (N) [3, 8, 9].
There are
28008,8 population Among them According to medical and statistic review
54 771,0 people have bronchial asthma diseases according to medical and
statistic review
A general
estimator method was used in this study for the processing of questionnaire
data to perform a complex and justified selection of modern, most effective and safe antiasthmatic drugs.
Material and
methods
Questioning
was carried out of the medical staff from the following institutions: the
Ministry of health of Republic of Uzbekistan Republic scientific allergology
centre, the Republican specialised scientifical and practical medical center of
phthisiology and pulmonology named after Sh. Alimov, the The Republican
specialized scientifical practical medical center of therapy and medical
Rehabilitation, the First clinic of the Tashkent medical academy second clinic,
Tashkent medical academy 1-st hospital, the Healthcare chief direction of
Tashkent city, the Municipal hospital of Urgent medical aid, the Municipal
hospital №1 after Ibn Sino.
Drugs were
assigned to 3 classes according to recommendations of qualified
physician-experts. Experts answered questions containing drug names (trade and
international names, forms and doses). A three-score system was used for the
assessment of drugs: 3 scores were given to vitally important and life quality
improving drugs (drugs necessary for life saving and maintenance); 2 scores to
essential preparations (effective for the treatment of less dangerous but
serious diseases); 1 score to non-essential, secondary preparations (for mild
disease, doubtful efficacy, expensive drugs with symptomatic indications).
Drug
consumption indices were studied in the Republican Specialized Allergy Research
Center for 2005-2009 years. One hundred and two drugs for bronchial asthma were
selected for the VEN-analysis. Ninety one physician-experts participated in the
questioning.
One of the
study aims was to determine a competency level of specialists giving expert
assessment of drug use efficacy. Intellectual data analysis methods were used
for general estimation of the competency level of specialists in distributing
drugs to VEN groups.
A problem was
stated of calculating aggregated, general estimation parameters without evident
measure units, such as severity of the disease, competency level, etc. In
calculation of general estimators by methods of intellectual data analysis based
on the technology of neuron networks, information was used from
“object-property” tables. In our case objects were drugs, signs (features) –
their classification by experts in VEN-analysis [2, 4, 10].
Mathematic
statement of the problem was formulated as following.
The
fixed set of objects![]() , contains representatives of two disjoint classes
, contains representatives of two disjoint classes![]() .The objects were described using
.The objects were described using ![]() nominal signs
(features) (expert answers), each of those having 3 grades – 1, 2, 3 (according
to VEN groups, respectively).
nominal signs
(features) (expert answers), each of those having 3 grades – 1, 2, 3 (according
to VEN groups, respectively).
The necessity
of two-class recognition task solution is related to the fact that any general
assessment of parameters is relative. Objects of each class are opposed to
objects from the opposite class (for example, class represented by expert
assessment of drugs by allergologists and pulmonologists, to that by
therapists);
For our case general
estimator of object S was calculated by the formula:
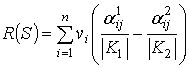 .
.
Where ![]() – the number of j-th
gradation of i-th sign (features) in the description of object S
in classes K1 and K2, respectively;
– the number of j-th
gradation of i-th sign (features) in the description of object S
in classes K1 and K2, respectively;
![]() – weight determined by interclass difference and innerclass
similarity of i-th sign (features) values;
– weight determined by interclass difference and innerclass
similarity of i-th sign (features) values;
![]() - the number of objects in
class
- the number of objects in
class ![]()
The drugs
were preliminary assigned to VEN groups taking into account graded assessment
of experts (K0 column, Table 2).
Three independent experiments were carried out
to calculate general estimator of drug assignment to class ![]() as opposite to class
as opposite to class![]() , to assess the expert competence in classification. In
the first experiment drugs from V group were classified into class
, to assess the expert competence in classification. In
the first experiment drugs from V group were classified into class ![]() with remaining ones
into
with remaining ones
into![]() . General estimator of assigning drugs to class
. General estimator of assigning drugs to class![]() , presented in [0, 1] scale, is given in V column of Table 2,
where 1 denotes absolute assignment of the drug to the class. In the second and
third experiments drugs of E and N groups were classified to class
, presented in [0, 1] scale, is given in V column of Table 2,
where 1 denotes absolute assignment of the drug to the class. In the second and
third experiments drugs of E and N groups were classified to class ![]() (E and N columns,
table 2).
(E and N columns,
table 2).
Results and
Discussion
Ninety one
questionnaires were distributed to specialists (allergologist, pulmonologist,
therapist) from the above institutions. Thirty six questionnaires were
considered unsatisfactory and were resubmitted and reevaluated. Categories
given in the questionnaire were fully assessed.
Displays
information on 91 specialists, of them 23 allergologists, 44 pulmonologists, 24
therapists.
Characteristics of expert physicians and assessment of indices in scores
Characteristics
of experts Index Number of experts and score
Specialty:
allergologist 23; pulmonologist, 44; therapist 24.
Duration of
work in healthcare (years) up: to 5, 6; from 5 to 10, 16; from 10 to 20 26;
from 20 to 30, 28; above 30, 15.
Duration of
work in specialty (years) up: to 5, 12; from 5 to 10, 21; from 10 to 20, 29;
from 20 to 30, 22; above 30, 7.
Qualification
category: highest 52; first, 18; second, 1; without category 20.
Academic
degree: doctor of medical sciences, 4; candidate of medical sciences, 20;
without degree, 67.
Academic
rank: professor, 5; docent, 6; not ranked, 80.
Level of familiarity
with bronchial asthma: practical experience 8; practical experience and
theoretical knowledge of medications, 83.
Certification:
certified 83; non-certified 8.
The majority
of questioned participants were physicians of the highest category (57.1%).
Service record in the healthcare system was 20-30 years in 24.2% and more than
30 years in 7.7% of the physicians.
Table 1
Optimal assortment of drugs
for bronchial asthma – VEN
|
№ |
International names |
Trade names, dosage, packing |
Drug forms |
VEN-experiments, R(S) |
|||||||
|
Name of drugs |
K0 |
V-(E, N) |
E-(V, N) |
N-(V, E) |
Kr |
||||||
|
|
Glucocorticoids |
|
|||||||||
|
1 |
Dexamethasone |
Dexamethasone 4 mg/ml
1 ml №25 |
sol. for inj. |
V |
1.00 |
0.00 |
0.00 |
V |
|||
|
2 |
Dexamethasone – GT
0.4 % 1 ml №5 |
sol. for inj. |
V |
0.63 |
0.43 |
0.28 |
V |
||||
|
3 |
Beclometasone |
Beclazon ECO light breathing
250 мg/dose
200 |
aerosol |
V |
0.90 |
0.13 |
0.08 |
V |
|||
|
4 |
Beclazon ECO light breathing
100 мg/dose
200 |
aerosol |
V |
0.78 |
0.31 |
0.05 |
V |
||||
|
5 |
Beclazon ECO 250 мg/dose
200 doses |
aerosol |
V |
0.74 |
0.35 |
0.13 |
V |
||||
|
6 |
Beclazon ECO 100 мg/dose
200 doses |
aerosol |
V |
0.68 |
0.44 |
0.14 |
V |
||||
|
7 |
Prednisolone |
Prednisolone 30
mg/ml, 1 ml №3 |
sol. for inj. |
V |
0.78 |
0.34 |
0.01 |
V |
|||
|
8 |
Prednisolone 0.005 g
№100 |
tablet |
V |
0.61 |
0.45 |
0.24 |
V |
||||
|
9 |
Prednisolone Nycomed
25 mg/1 ml №50 |
sol. for inj. |
V |
0.61 |
0.40 |
0.35 |
V |
||||
|
10 |
Prednisolone 5 mg
№100 |
tablet |
V |
0.58 |
0.47 |
0.27 |
V |
||||
|
11 |
Fluticasone |
Flixotide evohaler
250 мg 60 doses |
aerosol |
V |
0.64 |
0.42 |
0.27 |
V |
|||
|
12 |
Flixotide evohaler
125 мg 60 doses |
aerosol |
V |
0.58 |
0.54 |
0.26 |
V |
||||
|
13 |
Dexamethasone |
Dexamethasone 0.5 mg
№10 |
tablet |
E |
0.24 |
0.96 |
0.34 |
E |
|||
|
14 |
Dexamethasone 0.4% 2
ml №10 |
sol. for inj. |
V |
0.43 |
0.79 |
0.18 |
E |
||||
|
15 |
Dexamethasone-Darnitsa
0.4% 1 ml № 5 |
sol. for inj. |
V |
0.55 |
0.64 |
0.13 |
E |
||||
|
16 |
Dexamethasone
phosphate 0.4 % 1 ml №10 |
sol. for inj. |
V |
0.55 |
0.63 |
0.11 |
E |
||||
|
17 |
Dexamethasone 0.4 % 1
ml №5 |
sol. for inj. |
V |
0.57 |
0.61 |
0.13 |
E |
||||
|
18 |
Methylprednisolone |
Solu-Medrol 125 mg /
1 ml |
sol. for inj. |
V |
0.34 |
0.67 |
0.52 |
E |
|||
|
19 |
Solu-Medrol 500 mg,
7.8 ml |
sol. for inj. |
V |
0.32 |
0.67 |
0.57 |
E |
||||
|
20 |
Methypred 4 mg №30 |
tablet |
V |
0.41 |
0.63 |
0.43 |
E |
||||
|
21 |
Triamcinolone |
Polcortolone 4 mg №50 |
tablet |
V |
0.41 |
0.67 |
0.38 |
E |
|||
|
22 |
Budesonide |
Budesonide forte 10
ml 200 doses |
aerosol |
V |
0.44 |
0.65 |
0.40 |
E |
|||
|
|
Budesonide Mite 10 ml |
aerosol |
V |
0.41 |
0.63 |
0.50 |
E |
||||
|
24 |
Methylprednisolone |
Solu-Medrol 40 mg / 1
ml |
sol. for inj. |
V |
0.39 |
0.53 |
0.58 |
N |
|||
|
Antiallergic medications |
|||||||||||
|
25 |
Aminophylline |
Euphyllinum 2.4% 10
ml №10 |
sol. for inj. |
V |
0.84 |
0.19 |
0.04 |
V |
|||
|
26 |
Salbutamol |
Ventolin 100 мg/200
doses |
aerosol |
V |
0.82 |
0.25 |
0.04 |
V |
|||
|
27 |
Salamol ECO 100 мg /
dose 200 doses |
aerosol |
V |
0.71 |
0.40 |
0.13 |
V |
||||
|
28 |
Salbutamol – GT 100 мg 200 doses
12 ml |
aerosol |
V |
0.60 |
0.56 |
0.19 |
V |
||||
|
29 |
*Fluticasone
propionate, Salmeterol xinafoate |
Seretide 250, 120
doses |
aerosol |
V |
0.66 |
0.43 |
0.19 |
V |
|||
|
30 |
Seretide 125, 120
doses |
aerosol |
V |
0.66 |
0.45 |
0.18 |
V |
||||
|
31 |
Seretide discus
50/250 мg
60 doses |
Powder in caps. |
V |
0.65 |
0.45 |
0.19 |
V |
||||
|
32 |
Fenoterol |
Berotec N 100 мg /10 ml 200 doses |
aerosol |
V |
0.63 |
0.51 |
0.12 |
V |
|||
|
33 |
Aminophylline |
Euphillin 2.4% 5 ml
№10 |
sol. for inj. |
V |
0.58 |
0.51 |
0.24 |
V |
|||
|
34 |
Theophylline |
Theophyl SR 300 mg
№30 |
capsule |
E |
0.27 |
1.00 |
0.27 |
E |
|||
|
35 |
Theophyl SR 100 mg
№30 |
capsule |
E |
0.29 |
0.88 |
0.35 |
E |
||||
|
36 |
Theopecum 0.3 g №50 |
tablet |
E |
0.31 |
0.83 |
0.37 |
E |
||||
|
37 |
Theotard 200 mg №40 |
capsule |
E |
0.36 |
0.75 |
0.36 |
E |
||||
|
38 |
Theophyl SR 200 mg
№30 |
capsule |
V |
0.51 |
0.59 |
0.21 |
E |
||||
|
39 |
Aminophylline |
Euphyllin – Н 200 5
ml №10 |
sol. for inj. |
V |
0.38 |
0.76 |
0.37 |
E |
|||
|
40 |
Euphyllin 0.15 g №30 |
tablet |
V |
0.38 |
0.67 |
0.46 |
E |
||||
|
41 |
Orciprenaline |
Asthmopent 20 ml 400
doses |
aerosol |
V |
0.33 |
0.73 |
0.50 |
E |
|||
|
42 |
Fenoterol,
ipratropium bromide * |
Berodual N 10 ml 200
doses |
aerosol |
V |
0.47 |
0.71 |
0.23 |
E |
|||
|
43 |
Berodual 20 ml |
for inhalation |
V |
0.47 |
0.68 |
0.24 |
E |
||||
|
44 |
Fenoterol |
Berotec Н 100 мg/dose
10 ml 200 doses |
aerosol |
V |
0.52 |
0.64 |
0.23 |
E |
|||
|
45 |
Salmeterol |
Serevent 25 мg/60 doses |
aerosol |
V |
0.52 |
0.63 |
0.26 |
E |
|||
|
46 |
Salbutamol |
Salbutamol 12 ml,
aerosol |
aerosol |
V |
0.55 |
0.62 |
0.22 |
E |
|||
|
Antiallergic medications |
|||||||||||
|
47 |
Bacteria lyohpilizate |
Cromoglin 20 mg/ml 15
ml |
spray |
E |
0.18 |
0.94 |
0.58 |
E |
|||
|
48 |
Ketotifen |
Ketotifen 1 mg №30 |
tablet |
E |
0.20 |
0.88 |
0.57 |
E |
|||
|
49 |
Ketotifen 0.001 №30 |
tablet |
V |
0.33 |
0.72 |
0.45 |
E |
||||
|
Immunomodulating medications |
|||||||||||
|
50 |
Bacteria lyohpilizate |
Broncho-Munal P 3.5
mg, №10 |
capsule |
E |
0.16 |
0.87 |
0.54 |
E |
|||
|
51 |
Broncho-Munal P 7 mg,
№10 |
capsule |
E |
0.23 |
0.82 |
0.45 |
E |
||||
|
|
Expectorants |
||||||||||
|
52 |
Butamirate cictrate |
Sinecod 200 ml |
syrup |
N |
0.01 |
0.99 |
0.82 |
E |
|||
|
53 |
Sinecod 200 ml |
drops |
N |
0.00 |
0.98 |
0.88 |
E |
||||
|
54 |
Bromhexin |
Bromhexin-REMEDY
0.008 g №10 |
tablet |
E |
0.12 |
0.99 |
0.61 |
E |
|||
|
55 |
Bromhexin – 4.4 mg/5
ml 60 ml |
syrup |
E |
0.18 |
0.86 |
0.66 |
E |
||||
|
56 |
Bromhexin 0.008 g №50 |
tablet |
E |
0.19 |
0.86 |
0.59 |
E |
||||
|
57 |
Bromhexin 0.008 g №10 |
tablet |
E |
0.22 |
0.84 |
0.54 |
E |
||||
|
58 |
Bromhexin–8 Berlin
Chemie 8 mg № 25 |
pill |
E |
0.24 |
0.83 |
0.52 |
E |
||||
|
59 |
Bromhexin 8 mg №20 |
tablets |
E |
0.22 |
0.83 |
0.54 |
E |
||||
|
60 |
Ambroxol |
Pulmoxol 30 mg 5 ml,
150 ml |
syrup |
V |
0.30 |
0.86 |
0.35 |
E |
|||
|
61 |
Ambroxol 0.03 №20 |
tablet |
V |
0.34 |
0.84 |
0.33 |
E |
||||
|
62 |
Ambroxol KMP 0.03 g
№20 |
tablet |
V |
0.37 |
0.79 |
0.31 |
E |
||||
|
63 |
Ambrosan 30 mg №20 |
tablet |
V |
0.43 |
0.72 |
0.21 |
E |
||||
|
64 |
Lasolvan 15 mg/ 5 ml
100 ml |
syrup |
V |
0.40 |
0.67 |
0.36 |
E |
||||
|
65 |
Аmbroxol 15 mg/5 ml,
100 ml |
syrup |
V |
0.47 |
0.64 |
0.29 |
E |
||||
|
66 |
Bromhexin,
levomenthol |
Bronchosan 25 ml |
drops |
E |
0.27 |
0.73 |
0.52 |
E |
|||
|
67 |
Aminophyllin, ammonia chloride, diphenhydramine menthol * |
Hydrilline 120 ml |
syrup |
N |
0.23 |
0.72 |
0.64 |
E |
|||
|
68 |
Salbutamol,
bromhexin, guaifenesin, menthol * |
Lorkof 100 ml |
syrup |
V |
0,35 |
0,68 |
0.43 |
E |
|||
|
69 |
Dextromethorphan,
diphenhydramine |
Hydrilline DM 120 ml |
syrup |
V |
0.27 |
0.67 |
0.62 |
E |
|||
|
70 |
Glaucin, ephedrine* |
Broncholytin 125 g |
syrup |
V |
0.28 |
0.65 |
0.58 |
E |
|||
|
Antibiotics |
|||||||||||
|
71 |
Midecamycin |
Macropen 400 mg № 16 |
tablet |
E |
0.09 |
0.97 |
0.68 |
E |
|||
|
72 |
Macropen 175 mg/5 ml
115 ml |
granule |
E |
0.10 |
0.96 |
0.70 |
E |
||||
|
73 |
Ceftazidime |
Ceftazidime 1 g |
pulv. for inj. |
E |
0.09 |
0.97 |
0.68 |
E |
|||
|
74 |
Cefazolin |
Cefazolin sodium salt
1 g № 5 |
pulv. for inj. |
E |
0.15 |
0.87 |
0.67 |
E |
|||
|
75 |
Ceftriaxone |
Loraxone 1000 mg |
pulv. for inj. |
V |
0.28 |
0.81 |
0.49 |
E |
|||
|
76 |
Cefotaxime |
Cefotac 1 g |
pulv. for inj. |
N |
0.09 |
0.79 |
0.82 |
N |
|||
|
77 |
Cefotaxime 1 g |
pulv. for inj. |
N |
0.19 |
0.67 |
0.81 |
N |
||||
|
78 |
Claforan 1,0 |
pulv. for inj. |
N |
0.25 |
0.60 |
0.68 |
N |
||||
|
79 |
Cefoperazone |
Cefobid 1 g |
pulv. for inj. |
N |
0.25 |
0.59 |
0.80 |
N |
|||
|
80 |
Cefazolin |
Cefasolin – AKOS 1 g |
pulv. for inj. |
N |
0.18 |
0.73 |
0.79 |
N |
|||
|
81 |
Cefamezin 1000 mg №1 |
pulv. for inj. |
N |
0.20 |
0.67 |
0.77 |
N |
||||
|
82 |
Cefazolin – GT 1 g |
pulv. for inj. |
N |
0.18 |
0.74 |
0.77 |
N |
||||
|
83 |
Cefazolin KMP 1 g |
pulv. for inj. |
N |
0.22 |
0.68 |
0.75 |
N |
||||
|
84 |
Cefazolin 1 g |
pulv. for inj. |
N |
0.20 |
0.69 |
0.74 |
N |
||||
|
85 |
Cefazolin Teva 1 g |
pulv. for inj. |
E |
0.20 |
0.77 |
0.70 |
N |
||||
|
86 |
Ceftriaxone |
Ceftriaxone 1 g №1 |
pulv. for inj. |
N |
0.23 |
0.65 |
0.79 |
N |
|||
|
87 |
Ceftriaxone 1 g |
pulv. for inj. |
V |
0.30 |
0.53 |
0.75 |
N |
||||
|
88 |
Ceftriaxone – KMP 1 g |
pulv. for inj. |
V |
0.29 |
0.56 |
0.67 |
N |
||||
|
89 |
Azithromycin |
Azithromycin 0.25 №6 |
capsule |
V |
0.26 |
0.58 |
0.70 |
N |
|||
|
Synthetic antibiotics |
|||||||||||
|
90 |
Ciprofloxacin |
Ciprofloxacin 250 mg, №10 |
tablet |
N |
0.11 |
0.83 |
0.79 |
E |
|||
|
91 |
Cipro –500 mg №10 |
tablet |
N |
0.13 |
0.82 |
0.80 |
E |
||||
|
92 |
Sispres 250 mg № 14 |
tablet |
N |
0.16 |
0.75 |
0.73 |
E |
||||
|
93 |
Ofloxacin |
Tarivid 200 mg №10 |
tablet |
N |
0.09 |
0.67 |
1.00 |
N |
|||
|
94 |
Ofloxacin 200 mg №10 |
tablet |
N |
0.18 |
0.66 |
0.82 |
N |
||||
|
95 |
Ciprofloxacin
|
Ciprox 100 ml |
sol. for inj. |
N |
0.14 |
0.77 |
0.81 |
N |
|||
|
96 |
Ciproflaxacin 0.2 % 100 ml |
sol. for inj. |
N |
0.14 |
0.76 |
0.81 |
N |
||||
|
97 |
Ciprinol 250 mg № 10 |
tablet |
N |
0.13 |
0.79 |
0.81 |
N |
||||
|
98 |
Ciprolox 500 mg № 10 |
tablet |
N |
0.11 |
0.82 |
0.80 |
N |
||||
|
99 |
Ciflox 500 mg №10 |
tablet |
N |
0.14 |
0.76 |
0.79 |
N |
||||
|
100 |
Cipro –250 mg №10 |
tablet |
N |
0.16 |
0.78 |
0.79 |
N |
||||
|
101 |
Ciprox 250 mg №10 |
tablet |
N |
0.19 |
0.71 |
0.77 |
N |
||||
|
102 |
Ciflox 250 mg № 10 |
tablet |
N |
0.17 |
0.73 |
0.77 |
N |
||||
*Note: combined medications: solution for inhalation;
powder in capsules for inhalation; solution for
injection; lyophilisat for solution of
injections (bottles); in set with water dilution for
injections 7,8 ml, 15,6 ml (bottles); powder for
solution of injections per 1 g (bottles); solution
for injection 0,2 % per 100 ml (plastic bottles); solution
for injection per 100 ml.
After
treating questionnaire data taking into account scores of competency level 102
preparations were assigned to groups V - 55 (53.92%), E – 21 (20.59%) and N –
26 (25.49%) (K0 column, Table 1).
Drug
redistribution was performed using a maximum principle by general estimators in
VEN groups. It was found that of 102 preparations used in bronchial asthma 21
(20.59%) were assigned to group V, 56 (54.90%) to group E, 25 (24.51%) to group
N (column k, Table 1).
Based on the findings “Beclazon ECO light
breathing 250 мg/dose
200 dose aerosol” was distributed to groups V, E, N with coefficients 0.90,
0.13 and 0.08, respectively.
Table 1
listed 21 preparations assigned to group V with their proportion close to 1,
that is, insignificant. “Dexamethasone 4 mg/ml 1 ml №25 ampoule”
had the greatest share in group V followed by “Prednisolon 30 mg/ml, 1 ml №3”,
“Prednisolon Nycomed 25 mg/ml №50, “Dexamethasone – GT 0.4% 1 ml №5”.
Glucorticoid preparations used in the treatment of bronchial asthma such as
“Beclazon ECO light breathing 250 мg/dose 200 dose aerosol”, “Beclazon ECO light
breathing 100 мg/dose
200 dose aerosol”, “Beclazon ECO 250 мg/dose 200 dose aerosol”, “Beclazon ECO 100 мg/dose 200
dose aerosol”, “Flixotide evohaler 250 мg 60 dose
aerosol had higher indices and were recommended to group V. Preparations used
only for a relief of bronchial asthma attacks such as “Ventolin 100 мg / 200 dose
aerosol”, “Salamol ECO 100 мg/dose 200 dose aerosol”, “Salbutamol-GT 100 мg 200 dose 12
ml aerosol”, Euphyllin 2.4% 10 ml №10” ampoule” had also high indices and were
assigned to group V. Combined broncholytic preparations such as “Seretide 250,
120 doses”, “Seretide 125, 120 doses”, “Seretide discus 50 / 250 мg 60 doses”
had lower indices and were assigned to group V.
Drug form and
tablets’ dose under the international name Prednisolon, dealing with
glucocorticoids enter group V. Differences between two producers are 0,61 and
0,58, and, ampules are 0,78 and 0,61 accordingly.
Due to drug
form under international name dexametason is differed between four producers,
dealing with group E, and, they are from 0,79 to 0,61; and, differences between
three trade names of salbutamol, dealing with group E, are determined in terms
from 0,82 to 0,60. Differences by ketotifen,having the same international and
trade name, and dealing with group E, are determined from 0,88 to 0,72.
By the study
results at process of treatment bronchial asthma the importance of aerosol
drugs’ use, dealing with groups of glucocorticoids and broncholiths, were
determined. Aerosol form of drugs have necessary for life significance at
treatment bronchia asthma. And at treatment severe form of this disease in
hospital conditions the high efficacy has
the solution for injection dexematason 4 mgr /1ml. Use antibacterial
synthetic substances showed their non-effecacy.
Allergologists
were more competent in prescribing preparations for the treatment of bronchial
asthma.
In 2005–2009 the amount of using drugs at
treatment bronchial asthma are determined in us dollars.
Fig. 1 shows
the number of preparations used in 2005-2009 years with their distribution to
ABC groups. It was found that the number of preparations in groups A and B was
decreased. In 2009 preparations of group C accounted for 65.6%. Preparations
used in the treatment of allergic rhinitis, medical items for the treatment of
concurrent diseases, preparations purchased in the Republican Specialized
Allergologic Research Center were excluded from ABC-analysis. At the first stage
of our pharmacoeconomic study of drug consumption, ABC-analysis was carried out
of preparations for bronchial asthma used in
the Republican Specialized Allergologic Research Center for 2005-2009
years. In Table 2 drug consumption parameters were given in dollars and
percents. As it is seen usage of preparations (in sums) decreased in 2009
compared to 2005. In market economy conditions means allocated by the
government should be maximally used.
Table 4
Results of ABC and VEN
analyses (expenses for treatment of bronchial asthma,%)
|
Years |
АВС |
Groups |
|||||||
|
V |
E |
N |
Total |
||||||
|
2005 |
|
dollars |
% |
dollars |
% |
dollars |
% |
dollars |
% |
|
А |
11708,93 |
68,71 |
818,82 |
4,80 |
944,15 |
5,54 |
13471,90 |
79,04 |
|
|
В |
225,01 |
1,32 |
1141,71 |
6,70 |
1201,78 |
7,05 |
2568,50 |
15,07 |
|
|
С |
237,86 |
1,40 |
560,84 |
3,29 |
201,99 |
1,19 |
1000,70 |
5,88 |
|
|
|
12171,80 |
71,43 |
2521,37 |
14,89 |
2347,92 |
13,78 |
16464,82 |
100 |
|
|
2006 |
А |
8621,71 |
72,08 |
310,16 |
2,60 |
390,23 |
3,26 |
9322,10 |
77,94 |
|
В |
476,82 |
3,99 |
709,88 |
5,93 |
507,16 |
4,24 |
1693,86 |
14,16 |
|
|
С |
210,40 |
1,76 |
462,56 |
3,87 |
271,46 |
2,27 |
944,42 |
7,90 |
|
|
|
9308,93 |
77,83 |
1482,61 |
12,40 |
1168,85 |
9,77 |
11960,39 |
100 |
|
|
2007 |
А |
7167,66 |
74,86 |
342,26 |
3,58 |
– |
– |
7509,92 |
78,44 |
|
В |
256,20 |
2,67 |
522,59 |
5,46 |
746,68 |
7,80 |
1525,48 |
15,93 |
|
|
С |
124,13 |
0,86 |
302,71 |
3,16 |
154,16 |
1,61 |
581,00 |
5,63 |
|
|
|
7547,99 |
78,39 |
1167,56 |
12,20 |
900,84 |
9,41 |
9616,39 |
100 |
|
|
2008 |
А |
7643,18 |
76,91 |
– |
– |
– |
– |
7643,18 |
76,91 |
|
В |
923,71 |
9,30 |
432,21 |
4,35 |
306,24 |
3,08 |
1662,16 |
16,73 |
|
|
С |
284,22 |
2,86 |
188,57 |
1,19 |
159,12 |
1,60 |
631,92 |
5,65 |
|
|
|
8851,11 |
89,07 |
620,78 |
5,54 |
465,36 |
4,68 |
9937,27 |
100 |
|
|
2009 |
А |
8683,44 |
78,28 |
– |
– |
– |
– |
8683,44 |
78,28 |
|
В |
564,69 |
5,09 |
1018,04 |
9,17 |
316,91 |
2,85 |
1899,64 |
17,11 |
|
|
С |
92,22 |
0,83 |
312,47 |
2,82 |
105,14 |
0,95 |
509,83 |
4,61 |
|
|
|
9340,35 |
84,20 |
1330,51 |
11,99 |
422,05 |
3,80 |
11092,91 |
100 |
|
Note. Sum to
USA dollar exchange rate was taken from data of the Central Bank of Uzbekistan
for 2005-2009 years [1].
In 2005,
according to ABC-analysis, consumption of “Cephamezin 1000 mg №1” and “Serevent
25 мkg/60
doses” came to 5.54% and 4.80%, respectively. Both the preparations belonged to
group A.
But these
preparations, by importance, fall into groups E and N. According to the
findings, preparations of group V should be purchased first.
Consumption was determined of drugs rated in
group V according to ABC-VEN analysis used only for the therapy of bronchial
asthma for years 2005 – 68.71%, 2006 – 72.08%, 2007 – 74.86%, 2008 – 76.91% and
2009 – 78.28%.
These are
vital medications for bronchial asthma that should be purchased by population
and medical institutions in future. A list was compiled of necessary secondary
medications used for the treatment of the disease. In future medical
institutions should properly distribute their funds in purchasing medications.
Conclusions
Based on methods of intellectual data
processing, general estimators were obtained for assignment of drugs to VEN
groups with determination of specialist competence level in their classifying.
Scores in questionnaires for distribution of
medications to VEN groups were treated by a modern intellectual technique to
assign preparations with highest indices into group V. A list was also given of
medications of groups E and N.
In future medial-preventive institutions
specialized in treating bronchial asthma should really estimate their
potentialities in obtaining vitally necessary medications.
Main medications used in treatment of bronchial asthma belong to groups
E and N.
A list was compiled of medications effective for recovery of life
quality and physical condition of asthmatic patients.
Based on study results, population and
medical-preventive institutions will be given an opportunity for rational use
of their resources.
Literature
1.
Валютная
политика // Экономика Узбекистана: Информационно-аналитический обзор
за 2008 год. – Ташкент, 2009. – С. 23 – 24.
2.
Игнатьев
Н. А., Мадрахимов Ш. Ф. О некоторых способах повышения прозрачности нейронных
сетей // Вычисл. технологии. – Ташкент, – 2003. – Т. 8 – № 6. – С. 31 – 37.
3.
Коленчик
О. В., Бреднева Н. Д., Зевакова В. А. Фармакоэкономические исследования
лекарственного обеспечения больных рассеянным склерозом // Фармация. – 2007. –
№ 6. – С. 23 – 25.
4.
Мадрахимов Ш. Ф., Хуррамов А. Х. Умумлашган
кўрсаткичлар тегишлилик функцияси қийматлари сифатида //
Узб. журнал «Проблемы
информатики и энергетики». – Ташкент: Фан, 2009. – № 6. – С. 82 – 87.
5.
Назаров Ж. А. Эпидемиологические аспекты
бронхиальной астмы и вопросы оптимизации фармакотерапии // Материалы
Республиканской научно-практической V-конференции: «Современные проблемы
диагностики, лечения и профилактики аллергических заболеваний», посвященной
100-летию аллергологии. 17 ноября 2006. – Ташкент, 2006. – С. 49–52.
6.
Овчаренко С. В., Передельская О. А.,
Аксельрод А. С., Морозова Н. В. Опыт применения небулайзерной терапии в лечении
больных тяжелой бронхиальной астмой // Клиническая медицина. – 2002. – № 2. –
С. 63 – 66.
7.
Пронина С. А.,
Омельяновский В. В., Баркова Ю. В. Моделирующие исследования как метод
фармакоэкономического анализа. Создание модели течения заболевания на примере бронхиальной
астмы // Иммунология. – 2003. – № 3. – С. 158 – 161.
8.
Родина
Ю. С., Кныш О. И. Методы фармацевтического маркетинга и составление перечня
современных средств контрацепции // Фармация. – 2007. – № 4. – С. 28–30.
9.
Тарасенко Е.В. Фармакоэкономическая оценка
эффективности лечения гастроэзофагеальной рефлюксной болезни // Сучасна
гастроентрологiя – 2006. – № 6 (32). – С. 18–22.
10.
Adilova
F. T., Ignat’ev N. A., Madrakhimov Sh. F. The Approach to Individualized
Teleconsultations of Patients with Arterial Hypertension
// Global Telemedicine and Health Updates: Knowledge Resources. – 2010. – Vol.
3. – P. 372 – 376.
Authors’ data
Suyunov Nizom Davurovich, Doctor of philosophy
(Ph. D) pharmaceutical sciences, professor-assistant of
the Department of Pharmacy Organization of the Tashkent Pharmaceutical
Institute. Home address: 13/37, Cherdantseva street 1, Mirzo-Ulugbek district,
Tashkent. Post-code 100170.
Madrakhimov Shavkat Fayzullaev,
candidate of physico-mathematical sciences, docent of the Department of
Programmed and Network technologies of the Uzbekistan National University after
Mirzo Ulugbek.
Ikramova Guzal Makhamadzhanovna,
professor-assistant, Doctor of philosophy (Ph. D) pharmaceutical sciences,
docent of the of the Department of Pharmacy Organization of
the Tashkent Pharmaceutical Institute.
Address: 40, Aybek street, Mirabad district, Tashkent.
1. Suyunov
Nizom Davurovich, the 3-d-year-doctorant of the Chair of Organization of the
Pharmaceuticsm.
Suyunov
Nizom Davurovuch Tashkent Pharmaceutical Institute
With
great gratitude, Doctorant of the Chair of Organization of the Pharmaceutics. Tashkent.
Pharmaceutical Institute.
Oybek Street, 40. Mirabad District,Tashkent. Uzbekistan.
1.
Суюнов
Низом Давурович, докторант
III курса кафедры «Организация фармацевтического дела» Ташкентского
фармацевтического института. Домашний адрес: город Ташкент, Мирзо-Улугбекский
район, ул. Черданцева 1, дом 13, квартира 37. Индекс 100 170.
2.
Икрамова
Гузал Махамаджановна, ассистент, соискатель кафедры «Организация
фармацевтического дела» Ташкентского фармацевтического института.
3.
Мадрахимов
Шавкат Файзуллаевич, кандидат
физико-математических наук, доцент кафедры «Программные и сетевые технологии»
Национального университета Узбекистана имени Мирзо Улугбека.
Республика
Узбекистан, г.Ташкент, Ташкентский фармацевтический институт, г.Ташкент,
Мирабадский район, ул. Ойбека, 40.