Физика/Применение физических методов в
медицине
PhD, T. Y. KUZNETSOVA
Yu. Kondratyuk Poltava National
Technical University, Ukraine
PhD, N. V.
SOLOVYOVA,
Higher State Medical Educational Institution of
Ukraine Ukrainian
Medical Stomatological academy, Poltava, Ukraine
the study of
anti-radical activity of endooxidant when interacting with free radicals
To decrease the negative effect of free oxygen
radicals on a living organism practical medicine widely uses endogenous
oxidants since they take part in the system of human organism protection from
the aggressive action of free radicals, for example [1-2]. The lack of
systematic investigations, especially at the molecular level, of antiradical activity
of various antioxidants under their interaction with free radicals in
biological systems not only determines availability of contradictory estimates
in interpretation of the results of experimental regularities [3-5] but also
creates difficulties in development of general ideas concerning the mechanisms
of interaction of antioxidants with free radicals and purposeful approach to
the control of these processes which are applied to medical practice [6, 7].
The above said actualizes studying the antiradical activity of various
antioxidants.
Interaction of antioxidants with free radicals
is determined by the influence of the great number of various interrelated
kinetic processes which stabilization is rather problematic even in the
experiment conditions. Thus, it seem urgent to study efficiency of the
influence of endogenous antioxidants by simulating the mechanism of their
interaction with free radicals by the methods of quantum chemistry in
combination with experimental ones, in particular, with electrochemical method
that allows not only obtaining the substantiation of the positive effect of
using the antioxidants but also establishing potential significance of these
substances as medical remedies.
The work objective was investigation of antiradical
properties of endogenous antioxidant glutathione (C10H17N3O6S)
by simulation of the mechanism of its interaction with free radicals (hydroxyl
radical (•OH) and superoxide-anion-radical (•OO-
).
Materials and methods
Human organism contains a nonenzymatic antioxidant
system of cells protection from the influence of free radicals. The compounds
with various properties appear as the system components. One of such compounds
is glutathione (GSH) [8] synthesized in each organism cell, but antiradical mechanism
of its interaction with active oxygen forms at the microscopic level is not
completely understood, except for certain results of macroscopic medical [9]
and electrochemical [10] investigations which are unfortunately of
phenomenological character and do not give a purposeful approach to such
processes control.
One of the key active forms of oxygen is •OO-
, which is formed when adding one electron to oxygen molecule in the basic
state and can be a source of •OH formation in human organism; it may be the
strongest oxidizer among free oxygen radicals [11], thus •OH and •OO-
can exist simultaneously and be used for studying their interaction with
glutathione for simulation of its antioxidant activity. The above said has
determined the choice of investigation objects.
Theoretical
study of the mechanism of GSH interaction with •OO-
and •OH is performed with the help of the program module GAMESS (version of
March 27, 2007) and program module Firefly 8 by the most modern unempirical
quantum chemical method in the basis 6-31G** [12].
Results and Discussion
When GSH molecules interact with one •OO-
at the point of global minimum of full interaction energy there occurs
redistribution of the charge of 702e with •OO-
to glutathione molecule through the atom of hydrogen H(23), indicating a
possibility of efficient interaction of •OO-
with GSH, with probable formation of stable complexes (Fig. 1). Under
analogous interaction with one •OH, on the contrary, there occurs an increase
of electron density on oxygen atom of hydroxyl radical by 0.208e, as a result
the bond length S(22) - H(23) increases in glutathione molecule from 0.132 to
0.317 nm that points to the possibility of this atom breaking off GSH
molecule and its further attachment to •OH with formation of water molecule
(Fig. 2).
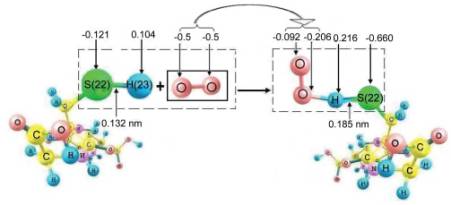
Fig. 1. Scheme of interaction of GSH molecule with •OO-
(arrows
point to charges on atoms according to Lyovdin)
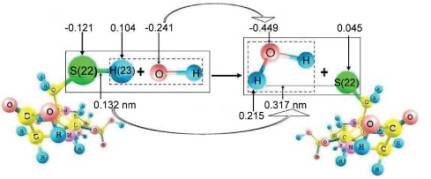
Fig. 2. Scheme of interaction of GSH molecule with •OH (arrows
point to charges on atoms according to Lyovdin)
Thus, the interaction of a molecule of studied
antioxidant with free oxygen radicals initiates redistribution of electron
density in the glutathione molecule in different directions (Fig. 3) [13].
Fig.
3. Scheme of redistribution of electron density of GSH molecule as a result of
interaction with radicals
To bring the results of quantum-chemical
modeling closer to real conditions of interaction of the antioxidant molecule
with •OH and •OO-
in human organism the authors performed simulation of water medium influence
on the mechanism of GSH molecule interaction with free oxygen radicals in terms
of Firefly 8 program. An analysis of results obtained has shown that the
mechanism of electron density redistribution with allowance for water medium
influence with dielectric constant e = 78.355 at T = 298 K within the continual
model of the solvent PCM for these interactions remains almost unchanged,
that is confirmed by comparison of charges distribution according to Lyovdin,
corresponding distances in GSH, •OH, •OO-,
as well as the values of activation energy of the reactions of GSH molecule
interaction with •OH and •OO- (Table).
Table
Comparative distribution of charges q according to Lyovdin
and activation energies Ea under GSH molecule interaction with free
oxygen radicals at a point of global minimum
|
Interaction |
q, a.o. |
Ea , kJ/mol |
||||
|
S(22) |
H(23) |
O* |
||||
|
GSH |
•OH |
Without PCM |
0.045 |
0.215 |
-0.449 |
101 |
|
PCM |
0.036 |
0.222 |
-0.465 |
100 |
||
|
•OO- |
Without PCM |
-0.660 |
0.216 |
-0.206 |
17 |
|
|
PCM |
-0.731 |
0.211 |
-0.187 |
7 |
||
*Indicated atom of radical which directly interacts with atom H(23) of
GSH molecule.
Thus, the quantum chemical simulation of glutathione
molecule interaction with •OH and •OO-
has shown that,
allowance for the influence of water medium do not practically influence
redistribution of electron density of glutathione
molecule and permit concluding that the studied reaction proceeds following the
acid-base mechanism, under these conditions GSH appears as acid in respect of
•OH in accordance with the set scheme (Fig. 3).
Thus, the
mechanism of glutathione molecule interaction with •OH and •OO-
has been investigated.
References
1.
Чеснокова Н.П., Понукалина Е.В., Бизенкова М.Н.
Молекулярно-клеточные механизмы инактивации свободных радикалов в биологических
системах //Успехи соврем.
естествознания − 2006.− № 7. − С.29–36.
2.
Boveris A. Determination of the production of superoxide
radicals and hydrogen peroxide in mitochondria.// Methods Enzymol.– 1984. –105.
– p. 429-435.
3.
Магин Д.В., Измайлов Д.Ю., Попов И.Н., Владимиров Ю.А.
Фотохемилюминесценция как метод изучения антиоксидантной активности в
биологических системах. Математическое моделирование // Вопр. мед. xим.–
2000.– 46, №4.– С. 61-66.
4.
Ehlenfeldt M.K., Prior R.L. Oxygen radical absorbance capac
ity (ORAC) and phenolic and anthocyanin concentrations in fruit and leaf
tissues of highbush blueberry // Journal of Agricultural and Food Chemistry.–
2001. – № 49. –P. 2222–2227.
5.
Korotkova E.I., Karbainov Y.A., Avramchik O.A. Investigation
of antioxidant and catalytic properties of some biologically active substances
by voltammetry// Anal and Bioanal
Chem.– 2003.– 375, № 1-3.–P. 465-468.
6.
Бачурин С. О. Медико-химические подходы к направленному
поиску препаратов для лечения и предупреждения болезни Альцгеймера //Вопр. мед.
хим.–2001, 47, № 2.– С. 155-197.
7.
Prutz W.A., Butler J., Land E.J. The glutathione free
radical equilibrium mediating electron transfer to Fе(III) – cytochrome //J. Biophys. Chem. – 1994.–
49(2). – Р.101–111.
8.
Кулинский
В.И., Колесниченко В.И. Биологическая роль глутатиона // Успехи
современной биологии. – 1990. –51,
№1(4).– С. 20–33.
9.
Anderson M.E. Glutathione: an overview of biosynthesis and
modulation // Chem. Biol. Interact. 1998. –111–112. – P. l–14.
10.
Шаповал Г.С. , Миронюк И.Е., Громовая
В.Ф., Кругляк О.С. Электрохимическое моделирование
редокс–реакций глутатиона // Журнал общей химии.– 2008. – 78, № 12. –
С. 2040–2044.
11.
Колісник М. І., Колісник
Г. В., Нідзюлка Є., Влізло В. В. Активні форми кисню та їх роль у метаболізмі
клітин // Біологія тварин. — 2009. –
11, № 1–2. – С. 59–67.
12.
Alex A. Granovsky. Firefly and PC GAMESS /Firefly version 8.0.1. [Electronic
resource]. − Access mode //http://classic.chem.msu.su /gran/games/forum/
discussion.html
13.
Кузнецова Т.Ю., Соловьева Н.В.
Моделирование антиоксидантных свойств мелатонина и глутатиона при
взаимодействии с гидроксил-радикалом // Актуал. пробл. суч.
мед.: Вісн. Укр. мед. стомат. акад.– 2012. – 12, № 1-2(37-38)
– С.189 – 193.