Õèìèÿ è õèìè÷åñêèå òåõíîëîãè / 5. Ôóíäàìåíòàëüíûå ïðîáëåìû
ñîçäàíèÿ íîâûõ ìàòåðèàëîâ è òåõíîëîãèé
Titova
Yu.V., Majdan D.A., Sholomova À.V.,
Aleksandrov D.Yu.,Khisamutdinova À.V.
Samara State Technical University
Preparation of aluminum nitride nanopowder using
SHS azide technology
A distinctive feature of nitride
compounds are broad areas of homogeneity where a great amount of vacant sites is
formed while their crystal structure is maintained. In addition, like many
other phases of implementation, nitrides are capable of forming numerous solid
solutions, many of which are distinguished by improved operating
characteristics.
The nitrides of elements of III
group (B, Al, Ga, In) and, particularly, aluminum nitride occupy a special
place among the nitride compounds. The application perspectiveness of AlN is
specified by the width of its band gap (6.28 eV), high value of the critical
electric breakdown, great heat resistance and mechanical strength [1]. Aluminum
nitride is the only ceramic material that has an extremely interesting
combination of high thermal conductivity and excellent insulation properties.
Due to this it is widely used in energetics and microelectronics. For example,
AlN is utilized in the manufacturing of printed circuit boards (substrates) in
semiconductors, it serves as a heat sink for the led lighting technology and in
high-power electronics. Moreover, aluminum nitride is viable for hardening
aluminum alloys operating at high temperatures.
Nowadays there is a huge number of
technologies for producing of aluminum nitride: direct nitriding,
plasma-chemical synthesis, carbothermic synthesis, chemical deposition from the
gas phase, the explosion of aluminum wire, etc.
This article [2] presents the
results of plasma-chemical synthesis of aluminum nitride nanopowder using
gaseous nitrogen and ammonia. A nanocomposite of Al/AlN with spherical
particles sized 50-100 nm is generated when pure nitrogen is used as a reaction
gas. Nitrogen content in the samples increases and the average particle size
decreases by adding ammonia.
Aluminum nitride was obtained by the
method of chemical deposition from the gas phase, using the system AlCl3-NH3-N2
[3]. Gaseous aluminum chloride, ammonia and nitrogen were reacted at a
temperature 1044 °C. The resulting powders’ particles have a spherical shape
and an average size of less than 0.1 micrometers.
In the following test [4] the
aluminum nitride was synthesized by the reaction of aluminum chloride and
sodium azide. The mixture was placed in a reactor with a nitrogen atmosphere,
and then the reactor was placed into a furnace for heating. The reaction
temperature of 450 °C was maintained for 24 hours. The synthesized product was
a micro-ribbon of AlN and bit long, straight fibers with a diameter of 40 to 60
nm and length of several micrometers.
The process of formation of aluminum
nitride in terms of electrical explosion of aluminum wire was investigated by
the authors of the present article [5]. As a result of the search for better
conditions of electrical explosion, a powder with a content of AlN equal to 93%
and a specific surface area of 14 m2/g.
However, these methods are
associated with large power inputs and high-cost and complex equipment is
required for them, the prepared powders have a strongly defect structure,
because of the shock cooling of the end-product. The self-propagating
high-temperature synthesis (SHS) of refractory compounds is free of these
disadvantages. It is characterized by low power inputs; short process duration;
high purity of products; the possibility to prepare new compounds, especially
multiphase composites, which are difficult to synthesize using other
technologies; and wide possibilities to control the dispersed structure of
powders from single-crystal grains to nanodimensional particles [6, 7]
The method is based on the
exothermic reaction of two or more chemical elements occurring in the
directional mode of combustion. The process is carried out in a thin layer of
the mixture of the initial agents after local initiation of the reaction
(ignition via electric impulse with a duration of 3-5 seconds) and
spontaneously propagates throughout the system due to heat transfer from the
hot products to original unheated materials that do not require energy input.
The authors [8] applied the SHS
method for the synthesis of highly dispersed AlN powder by combusting the
initial mixture of aluminum and gasifying additive (NH4Cl, NH4F) in the nitrogen medium. It is stated
that the degree of conversion of the aluminum to the nitride increases with an
increase in the proportion of aluminum in the charge and reaches a maximum
value (99.5 %) when the content of aluminum in the mixture in the amount of 50 wt
%. The particle diameter of the aluminum nitride decreases from 8-12 μm to about 1-2 μm, and the specific surface area increases up to 1.5 m2/g
with increasing of ammonium chloride proportion in the mixture up to 10 mass %.
Starting from 1970, the azide
technology of self-propagating high-temperature synthesis (SHS-Az) has been
developed at the Samara State Technical University. This technology makes it
possible to fabricate micropowders and nanopowders of nitrides and composites
based on them using the sodium-azide (NaN3) powder as the nitriding
reagent and haloid salts [9].
The goal of this study was to
investigate the possibilities of applying the SHS-Az technology to fabricate
the nanostructured aluminum nitride powder.
The stoichiometric equation of the
preparation reaction of aluminum nitride in the SHS-Az mode is as follows:
AlF3 + 3NaN3 =
AlN + 3NaF + 4N2, (1)
Na3AlF6 + 3NaN3
= AlN + 6NaF + 4N2. (2)
The usage of halide salts containing aluminum (AlF3,
Na3AlF6) instead of metallic aluminum as a starting component
reduces the combustion temperature and also enables the performing of the
reaction on the atomic level. In this case, it is possible to synthesize the
nanosized powders of aluminum nitride.
As the initial feedstock, we used the
sodium azide powder of the “Pure” grade (assay percentage 98,71 wt.%), aluminum fluoride
powder of the “Pure” grade (assay percentag 99,9 wt.%), sodium hexa-fluoroaluminate
powder of the “Pure” grade (assay percentag99,0wt.%), nitrogen gas quality “1” (assay percentag 99,99 wt.%).
The procedure of experimental
investigations in an SHS constant-pressure reactor with a volume of 4.5 L is described
in monographs [9]. The cylindrical samples (diameter – 30 mm, height – 45 mm)
of the initial powder mixture of the apparent density under an external
nitrogen pressure in a reactor of 4 MPa were combusted.
We investigated the phase and
chemical compositions, morphology, and particle size of combustion products.
The phase composition was determined using an ARL X’TRA automated X-ray
diffractometer. X’ray spectra were recorded using Cu radiation with continuous
scanning in angle range 2θ = 20°–80° with a rate of 2 deg/min. The surface
topography and morphology of powder particles were investigated using a
JSM-6390A scanning electron microscope.
To preliminarily analyze the
combustion temperature of the mixture of initial components and composition of
synthesis products, thermodynamic calculations were performed using the Thermo
computed program developed at the Institute of Structural Macrokinetics and
Problems of the Materials Science of the Russian Academy of Sciences
(Chernogolovka, Moscow region).
Table 1 presents the results of
thermodynamic calculations, which show the values of the adiabatic temperature,
reaction thermal effect, and composition of combustion products for the two
main compositions of the initial mixture, which differ in haloid salts.
Table 1 – Results of
thermodynamic calculations of combustion parameters
|
Composition no. |
Charge
composition |
Adiabatic
temperature [K] |
Reaction thermal effect [kJ] |
Synthesis
products [mole] |
||
|
AlN |
NaF |
N2 |
||||
|
1 |
AlF3+3NaN3 |
1682 |
–135 |
1 |
3 |
4 |
|
2 |
Na3AlF6+3NaN3 |
1269 |
–107 |
1 |
6 |
4 |
From the analysis of the presented
data for the compositions 1 and 2 follows, that the adiabatic
temperature and the thermal effect are higher while using aluminum fluoride as
a starting component than sodium hexa-fluoroaluminate. However, the yield of
the desired product - aluminum nitride is
the same in both speciations.
The results of the experimental
determination of the combustion temperature and its rate, as well as the phase
compositions under consideration, are presented in Table 2.
Table 2 – Combustion
parameters and composition of synthesis products
|
Composition no. |
Charge
composition |
Combustion
temperature[°C] |
Combustion
rate[mm/c] |
Phase
composition |
|
1 |
AlF3+3NaN3 |
1250 |
11 |
AlN, Na3AlF6,
NaF |
|
2 |
Na3AlF6+3NaN3 |
950 |
6 |
AlN, Na3AlF6,
NaF |
It appears from Table 2 that, while
using sodium fluoride the combustion temperature and its rate are higher, than by using sodium hexa-fluoroaluminate by 300 °Ñ è 5 mm/s correspondingly. The combustion products of both
speciations consist of three phases: aluminum nitride (AlN), sodium hexa-fluoroaluminate
(Na3AlF6) and sodium fluoride (NaF).
However, their ratio differs.
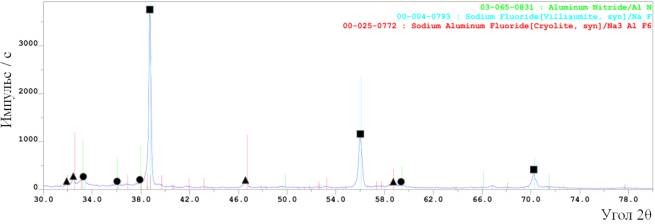
Figure 1 displays X’ray powder diffraction
patterns of synthesis flushed and unflushed products synthesized of the
speciation 1. the flushing was performed by diluting the
powders with distilled water in ratio
1:10, roiling the suspension and than filtrating the desired products in the
vacuum funnel. The filtered powder was blown dry to the constant weight in the
vacuum oven.
![]()
à)
b)
Figure 1 – The
results of X-ray phase analysis of the products, synthesized
of the mixture “AlF3+
NaN3”:
a)
before flushing; b)
after flushing
The X-ray
patterns demonstrate that the combustion produtcts of the temper “AlF3 + 3NaN3” consist of three phases:
aluminum nitride (AlN), sodium hexa-fluoroaluminate (Na3AlF6) and sodium fluoride (NaF). The peak
heights allow us to assume that NaF > Na3AlF6 > AlN.
The amount of the desired product - aluminum nitride is low, so that the X-ray pattern of the unflushed products there are no peaks of AlN.
A good solubility in water sodium fluoride is absolutely removed from the
combustion products after flushing in the distilled water. On the contrary, due
to bad solubility in water sodium hexa-fluoroaluminate exists in the combustion
roducts. The quantative phase composition
of the flushed combustion products of the temper “AlF3 + 3NaN3”
displayed the fraction of aluminum nitride – 64% and sodium hexa-fluoroaluminate – 36%.
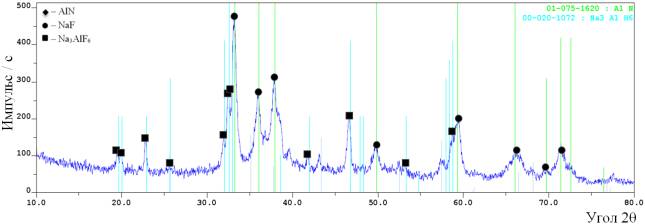
See figure 2. The X-ray
patterns of the unflushed and flushed produts synthesized form speciation
2.
à)
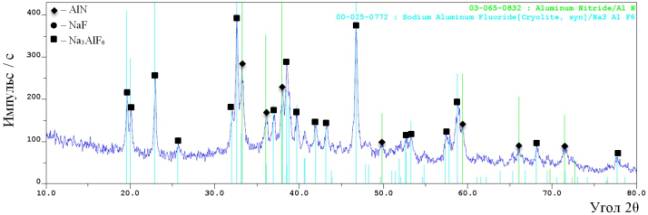
b)
Figure 2 – The
results of X-ray phase analysis of the products, synthesized
of the mixture
“Na3AlF6+ NaN3”:
a)
before flushing; b)
after flushing
The X-ray
patterns demonstrate that the combustion produtcts of the mixture
“Na3AlF6+ NaN3” consist of three phases:
sodium fluoride (NaF), sodium hexa-fluoroaluminate (Na3AlF6) and aluminum nitride (AlN). The peak
heights allow us to assume that NaF> Na3AlF6 >AlN.
The quantative phase composition of the flushed combustion products of the temper “Na3AlF6 + NaN3”
displayed the fraction of aluminum nitride – 61% and sodium hexa-fluoroaluminate – 39%.
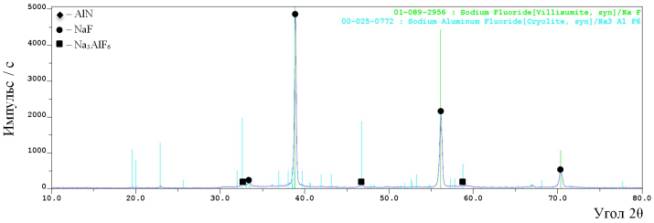
The process of flushing of sodium hexa-fluoroaluminate consisted of decomposing it into the fluorides of sodium and aluminum
which are soluble in water by heating them in Ar at a temperature of 400 °Ñ for 30 min and flushing it with water. This action allows extracting
the high purity aluminum nitride powder (figure 3).
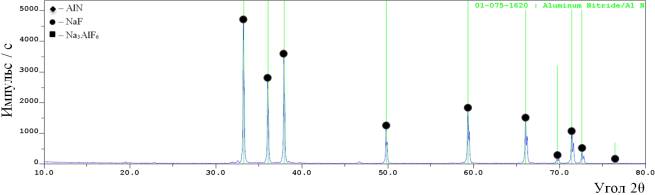
Figure 3 – The
results of X-ray phase analysis of the products, synthesized of the mixture
“AlF3+3NaN3” after been blown dry in the vacuum oven
See figure 3 we may see after been
dry in the vacuum oven the product consists of one phase - aluminum nitride.
See figure 4. The photographs of
microstructure of the aluminum nitride powders synthesized of speciation 1 and
2
|
|
|
|
à) |
b) |
Figure
4. The microstructure of synthesized aluminum nitride powders:
a)
“AlF3+3NaN3”; b)
“Na3AlF6 + NaN3”
The picture displays a crystal whiskers structure of aluminum
nitride, synthesized of the mixture “AlF3+3NaN3”. The
whiskers have a diameter from 100 to 200 nm and in length they are about 5 µm.
Aluminum nitride, generated in the combustion of the temper “Na3AlF6
+ NaN3” has a structure of the spherical particles with a diameter
from 50 to 140 nm.
The applying of energy-saving SHS
azide technology allowed extracting high purity aluminum nitride micro and
nanopowders of the speciations “AlF3+3NaN3” and “Na3AlF6
+ NaN3”. It is of tremendous value for using them in electronics and
electric engineering. AlN is synthesized as the crystallized whiskers with the
diameter 100-200 nm and spherical particles with the diameter 50-140 nm
depending on the haloid salt used in the reaction.
Bibliography:
1. Yu.A. Bystrov, N.Z. Vetrov, A.À. Lisenkov. Plasmachemical
synthesis of aluminum-based nitride compounds in vacuum-arc discharge plasma // Technical Physics Letters (2012) Volume
38.20. p. 50-56.
2. Kim K. Plasma synthesis and
characterization of nanocrystalline aluminum nitride particles by aluminum
plasma jet discharge // Journal of Crystal Growth 283 (2005) 540–546.
3. Wu N., Tsai M., Wang M., Liu H. /
The morphology and formation mechanism of aluminum nitride nanocrystals
synthesized by chemical vapor deposition // Journal of Crystal Growth 208
(2000) 189-196.
4. Wang, H.L. Synthesis of hexagonal
AlN microbelts at low temperature // Journal of Alloys and Compounds. – 2009. –
Ð. 580-582.
5. Beketov I.V. Electric explosion
of wires as a method for preparation of aluminum nitride nanopowder // The
Second Russian conference of nanomaterials «NANO 2007» (13-16 march 2007, Novosibirsk)
– p. 109.
6. Levashov, E.A., Rogachev, À.S., Kurbatkina, V.V. Perspective materials and SHS technologies. Moscow, (2011) 377p.
7. Amosov, A. P., Borovinskaya,
I. P., Merzhanov, À. G. Poroshkovaya. SHS Technology of material synthesis: Educational
Aid / Amosov, A. P, Edited V. N. Anficerov. – Moscow: Machinery
-1, (2007) – 567 p.
8. Zakorzhevsky, V. V., Borovinskaya,
I. P., Sachkova, N. V. Aluminum nitrides synthesis through combustion of the mixture
Al+AlN // Inorganic Material, 2002. – ¹ 11. – V. 38. – P. 1340-1350.
9. Amosov, A.P. Bichurov, G.V. SHS
azid technology of synthesis nitrides micro and nanopowders. Moscow: Machinery-1,
(2007) – 526 p.

