Õèìèÿ è
õèìè÷åñêèå òåõíîëîãèè/7. Íåîðãàíè÷åñêàÿ õèìèÿ
Bekturganova A.Zh.1, Rustembekov K.T.1, Stoev M.2,
Ybrahym S.A.1, Abdrahmanova D.S.1, Toyganbaeva D.Zh.1
1Ye.A.Buketov
2South-West
University «Neofit Rilski»,
Calorimetry of double zinc tellurite
Knowledge of the thermodynamic
properties of complex oxo compounds is necessary to create a data bank of
thermodynamic quantities of data, modeling of processes of synthesis of new
materials with desired characteristics and to identify the fundamental
dependence "structure - property" in the synthesized substances.
The aim of this work was calorimetric research of the heat capacity of double zinc tellurite. Tellurite
composition Na2Zn(TeO3)2 was synthesized
by ceramic technology from tellurium (IV) oxide, zinc and sodium carbonate. The
formation of the equilibrium composition of the compound was monitored by X-ray
diffraction.
The heat capacities of tellurite were studied via dynamic
calorimetry on an commercial IT-S-400 calorimeter in the temperature range of
298.15-673 K (Table 1). Errors in the measurement of the
heat capacity at all temperatures are within the accuracy of the instrument (±
10%).
Table 1
Experimental specific and molar heat
capacities of the Na2Zn(TeO3)2
|
Ò, Ê |
J/(g Ê) |
J/(mol Ê) |
Ò, Ê |
J/(g Ê) |
J/(mol Ê) |
|
1 |
2 |
3 |
4 |
5 |
6 |
|
298,15 |
0,4683±0,0162 |
217±21 |
498 |
0,6892±0,0043 |
319±6 |
|
323 |
0,5230±0,0045 |
242±6 |
523 |
0,6969±0,0052 |
322±7 |
|
348 |
0,6038±0,0039 |
279±5 |
548 |
0,5827±0,0097 |
270±13 |
|
373 |
0,4183±0,0108 |
193±14 |
573 |
0,6318±0,0030 |
292±4 |
|
1 |
2 |
3 |
4 |
5 |
6 |
|
398 |
0,4972±0,0077 |
230±10 |
598 |
0,6689±0,0043 |
309±6 |
|
423 |
0,5455±0,0065 |
252±8 |
623 |
0,6947±0,0071 |
321±9 |
|
448 |
0,6057±0,0086 |
280±11 |
648 |
0,7203±0,0110 |
333±14 |
|
473 |
0,6312±0,0067 |
292±9 |
673 |
0,7358±0,0051 |
340±7 |
For an average of the specific heat at each temperature was determined by standard deviations (![]() ), and for molar heat capacities -
random error components
), and for molar heat capacities -
random error components ![]() . Random error components experimental
values of heat capacities do not exceed the limits of the instrument error.
Operation of the calorimeter was tested by measuring the heat capacity of a-Al2O3. The resulting
. Random error components experimental
values of heat capacities do not exceed the limits of the instrument error.
Operation of the calorimeter was tested by measuring the heat capacity of a-Al2O3. The resulting ![]() (298,15) = 76.0 J/(mol Ê) was in
satisfactory agreement with the reference value (79.0 J/(mol Ê)).
(298,15) = 76.0 J/(mol Ê) was in
satisfactory agreement with the reference value (79.0 J/(mol Ê)).
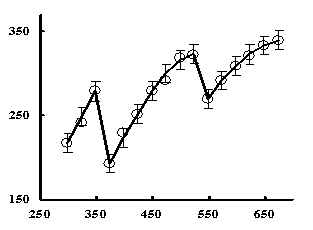
In studying the temperature dependences of the
heat capacities of Na2Zn(TeO3)2 was at 348 and
523 K, we observed abnormally sharp l-like
peaks probably associated with second-order phase transitions (Figure). Òhese
transitions could be due to the redistribution of cations, changes
in coefficient of thermal expansion and changes in the magnetic
moment of synthesized tellurite.
|
Ò, Ê |
||
|
Figure - The temperature dependence of heat capacity of tellurite Na2Zn
(TeO3)2 in the
range of 298.15 - 673 K |
Due to the presence of second-order
phase transitions, the dependence ![]() of
compound described by several equations the coefficients of which
are given in Table 2.
of
compound described by several equations the coefficients of which
are given in Table 2.
Table 2
Equations
of the temperature
dependence of heat capacity of the
Na2Zn
(TeO3)2 in the
range of 298.15 - 673 K
|
Compound |
Coefficients
of Equation J/(mol
Ê) |
|
||
|
a |
b·10-3 |
c·105 |
||
|
Na2Zn(TeO3)2 |
–156,8±5,5 |
1253,0±44,0 |
– |
298-348 |
|
1473,8±51,7 |
–3432,4±120,5 |
– |
348-373 |
|
|
682,3±24,0 |
–334,0±11,7 |
–506,7±17,8 |
373-523 |
|
|
1427,3±50,1 |
–2112,8±74,2 |
– |
523-548 |
|
|
918,1±32,2 |
–477,1±16,8 |
–1162,6±40,8 |
548-673 |
|
Using the experimental data on ![]() , and the estimated values of
, and the estimated values of ![]() , from the known ratio in the range of
298.15 – 673 K we calculated the temperature dependence of
functions
, from the known ratio in the range of
298.15 – 673 K we calculated the temperature dependence of
functions ![]() ,
, ![]() ,
, ![]() ,
, ![]() (Table 3).
(Table 3).
Table 3
Thermodynamic
functions of the Na2Zn
(TeO3)2
in the
range of 298.15 - 673 K
|
Ò, Ê |
J/(mol Ê) |
J/(mol Ê) |
|
|
|
1 |
2 |
3 |
4 |
5 |
|
298,15 |
217±8 |
288±9 |
– |
288±19 |
|
300 |
219±8 |
289±19 |
436±15 |
288±19 |
|
325 |
250±9 |
308±20 |
6306±221 |
289±19 |
|
1 |
2 |
3 |
4 |
5 |
|
350 |
282±10 |
328±21 |
12960±455 |
291±19 |
|
375 |
187±7 |
344±22 |
18698±656 |
294±19 |
|
400 |
232±8 |
356±23 |
24077±845 |
296±19 |
|
425 |
260±9 |
372±24 |
30236±1061 |
301±20 |
|
450 |
282±10 |
387±25 |
37016±1299 |
305±20 |
|
475 |
299±11 |
403±26 |
44283±1554 |
310±20 |
|
500 |
313±11 |
420±27 |
51938±1823 |
316±21 |
|
525 |
323±11 |
434±28 |
59887±2102 |
320±21 |
|
550 |
265±9 |
448±29 |
67179±2358 |
326±21 |
|
575 |
292±10 |
461±30 |
74233±2606 |
331±22 |
|
600 |
309±11 |
473±31 |
81754±2870 |
337±22 |
|
625 |
322±11 |
486±32 |
89651±3147 |
343±22 |
|
650 |
333±12 |
499±32 |
97846±3434 |
349±23 |
|
675 |
341±12 |
512±33 |
106273±3730 |
354±23 |
Thus, the isobaric heat capacities of double zinc tellurite were determined by dynamic calorimetry in the temperature range
298.15 - 673 K. Equations
describing their temperature dependences were derived. Dependences
for tellurite Na2Zn(TeO3)2 at 348 and 523 K were
found to have sharp abnormal peaks, l-like
effects attributable to a second-order phase transition. Thermodynamic functions
![]() ,
, ![]() ,
, ![]() ,
, ![]() were
calculated. The existence of a second-order phase transition in the curve of
the temperature dependence indicates that this compound could have unique
electrophysical properties.
were
calculated. The existence of a second-order phase transition in the curve of
the temperature dependence indicates that this compound could have unique
electrophysical properties.