Ôèçèêà / 2.Ôèçèêà òâåðäîãî òåëà
Titova Yu.V., Majdan D.A., Sholomova À.V.,
Aleksandrov D.Yu., Khisamutdinova À.V.
Samara State Technical University
Self-Propagating High-Temperature Synthesis
of Nanostructured AlN Powder with the Use of AlF3 and NaN3
One fundamental base of technological advance is the development of new
materials meeting demands of modern technology. In 1967, when studying gas-free
combustion of mixtures of powders of metals and nonmetals, Russian scientists
(Academician A.G. Merzhanov, Professor I.P. Borovinskaya, and Professor V.M.
Shkiro) in the academic borough of Chernogolovka near Moscow developed the new
synthesis of compounds, including nitrides, which was called self-propagating
high-temperature synthesis (SHS) [1].
In 1970, Professor V.S. Kosolapov of the Kuibushev Polytechnic Institute
proposed using powders of solid inorganic azides, the application of which
increases concentrations of reacting substances in the synthesis zone and
eliminates filtration difficulties, instead of gaseous nitrogen as the
nitrifying reagent in SHS [2]. This is the beginning of azide technology of
self-propagating high-temperature synthesis (SHS-Az).
The investigation into the synthesis conditions and properties of
aluminum nitride AlN is considered in numerous publications [3-9], the results
of which were the origin of its application in modern engineering. Aluminum
nitride has a forbidden band of 6.2 eV with the direct transitions, which is
close to dielectric. Its resistivity is very high (greater than 1011 Ω), whereas permittivity is very low. Because of these
properties, AlN is ideally appropriate for the use as a material of electronic
substrates or the package of integrated circuits. It also has a low thermal
expansion coefficient of 4.3 × 10–6 K–1 and a high
thermal conductivity of 320 W m–1 K–1,
which is three times higher than for aluminum oxide, while it is the most
valuable known ceramic material [10].
The aim of the given work is to investigate the process for obtaining
the nanostructured powder of aluminum nitride in the SHS mode at the excess
pressure of nitrogen using sodium azide and aluminum fluoride.
The stoichiometric equation for the chemical reaction of obtaining
aluminum nitride in the SHS-Az mode is the following:
AlF3 +
3NaN3 = AlN + 3NaF + 4N2. (1)
In this study we investigated
the combustion temperature and rate of the starting component mixture under
conditions of a laboratory reactor with a constant pressure [2] and the
chemical and phase compositions of combustion products as a function of the
pressure of gaseous nitrogen in the reactor, the relative density of the
starting mixture, and the sample diameter.
Based on the
obtained data and technological considerations related to the use of pressing
equipment, it is appropriate to use the apparent density of the charge for the
further investigations. It is evident from data that the combustion temperature
and rate of the AlF3 + 3NaN3 system increase
with increasing sample diameter D
attaining the maximum at D = 3 cm.
Exceeding this value causes filtering difficulties for the nitrogen supply into
the central part of the sample. External nitrogen participates at the after burning
stage, which is why the desired product with a high N content forms [11]. Based
on these data, we chose a sample diameter of 3 cm for the further investigation
of the AlF3 + 3NaN3 system.
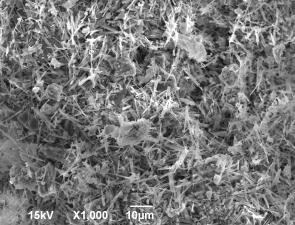
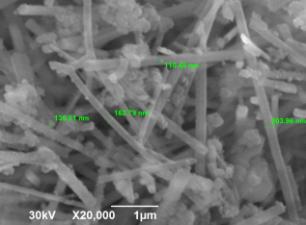
The surface
topography and particle sizes of the AlN powder synthesized from the AlF3 + 3NaN3 system are shown in Fig. 1. It is evident from the presented
photographs that aluminum nitride is synthesized in the form of particles
containing whiskers ~100 nm in diameter, which can be classified as nanofibers.
Data of X-ray
analysis (Fig. 2) testify that reaction products contain three phases: aluminum
nitride, sodium hexafluoroaluminate, and sodium fluoride. The last two
components are present only in unwashed combustion products and are removed by
washing in distilled water.
|
|
|
|
a |
b |
Figure
1 – Surface topography of AlN powders synthesized in the “AlF3 + 3NaN3”
system:
(a) ×1000 and (b) ×20000
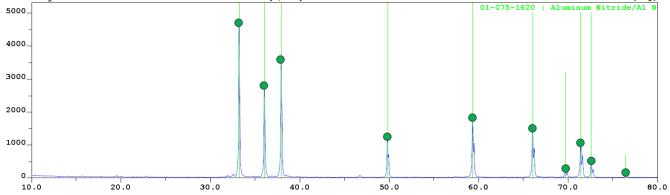
Figure
2 – X-ray diffraction pattern of the washed product synthesized
in the “AlF3 + 3NaN3”
system
Let us note that
the binary system that we investigated makes it possible for the first time to
synthesize the aluminum nitride powder in the form of nanofibers.
References
1. Amosov, A.P.,
Borovinskaya, I.P., and Merzhanov, A.G., Powder Technology of Self-Propagating
High-Temperature Synthesis of Materials: Moscow: Mashinostroenie-1, 2007.
2. Amosov, A.P.
and Bichurov, G.V. Azide Technology of Self-Propagating High-Temperature
Synthesis of Micropowders and Nanopowders of Nitrides, Moscow:
Mashinostroenie-1, 2007.
3. Kosolapov,
V.T., Shmel’kov, V.V., Levashev, A.F., and Markov, Yu.M., 2nd All-Russia Conf.
on Technological Combustion, Chernogolovka, 1978, pp. 129-130.
4. Prokudina,
V.K., Shestakova, T.V., Borovinskaya, I.P., et al., Problems of Technological
Combustion: Proc. 3rd All-Russia Conf. on Technological Combustion,
Chernogolovka, 1981, vol. 2, pp. 5-8.
5. Zakorzhevskii,
B.B., Borovinskaya, I.P., and Sachkova, H.B., Inorg. Mater., 2002, vol. 38, no.
11, p. 1131.
6. D’yachkov,
L.G., Zhilyakov, L.A., and Kostanovskii, A.B., Tech. Phys., 2000, vol. 45, no.
7, p. 928.
7. Borets-Pervak,
I.Yu., Kvantovaya Elektron., 1997, vol. 24, no. 3, pp. 265-268.
8. Grabis, Ya.P.,
Ubele, I.P., and Kuzyukevich, A.A., Latv. PSR Zinat. Akad. Vestis., Ser.
Khimiya., 1982, no. 3, pp. 279–282.
9. Titova, Yu.V.
and Shiganova, L.A., Proc. Int. Theor. and Pract. Conf. “Modern Innovations in
Science and Technology”, Kursk, 2011, pp. 113-115.
10. Samsonov,
G.V., Nitrides, Kiev: Naukova Dumka, 1969.
11. Bichurov,
G.V., Self-Propagating High-Temperature Synthesis of Nitrides with Application
of Inorganic Azides and Halogen Salts, Extended Abstract of Doctoral
Dissertation, Samara: Samara State Tech. Univ., 2003.