Panasenko A. I., Samura T. A., Buryak V.P., Gotsulya
A.S., Guzhva A.A., Vovnjanko O.I., Kulish S.N.
National University of the life and environmental
sciences of Ukraine
Zaporozhye State Medical University
Formation of ionized species
and hydrogen bonding of the some thiazoles and benzothiazoles.
The spectrum of
benzthiazoline-2-thione in solvents with different polarity is reported and a
solvent dependence of the charge-transfer band is noted. Blue shifts are
observed in hydroxylic solvents, where hydrogen bonding from solvent to the
thion sulfur atom occurs and shifts in solvents where the
benzotiazoline-2-thion functions as a hydrogen bond donor. In solvents where
the compound acts both as a donor and acceptor, are observed zero shifts.
These conclusions
are supported by the studies, in a range of critical solvents, of spectrum of
the N-methyl derivative, which can function as a hydrogen bond acceptor only,
and shows blue shifts only.
The spectra of
benzthiazoline-2-thione (I), 3(N)-methyl-benzthiazaoline-2-thione (II) and 9-Me
derivative (III) in concentrated sulfuric acid closely resemble with each other (Table I)
Table I
The spectra of I,
II and III in concentrated sulfuric acid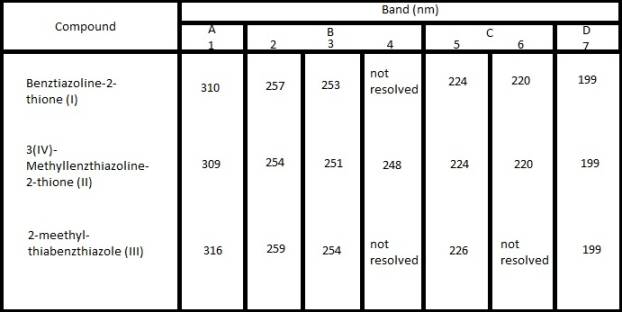
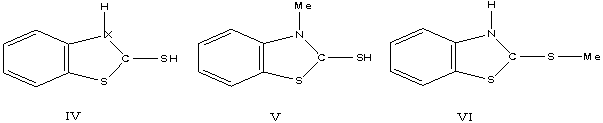
We attribute this
similarity to the formation of the singly-protonated species IV, V, VI, having
similar energy levels. The strong evidence for the protonation of the
double-bonded sulfur in thiamides is discussed by Talse and Orchin [6]. The
representation with partial charge on the sulfur and nitrogen is preferable to
that single positive charge on the nitrogen is discussed by West [12] for
methyl-benzthiazoline-2-thione. The more basic character at (III) is shown by
its protonation in dilute hydrochloric acid (233, 251, 259, 280, 290 nm). The
fact is that (I) in sodium hydroxide solution, in which the species present is
the ion VII, absorbs at approximately the same frequency as (I) in concentrated
sulfuric acid is fortuitous. All the negative charge would not residue on the
sulfur atom [7]. As expected the spectra of II
and III, in sodium hydroxide solutions are almost identical with these in
water.
The effects of
solvent an electronic transitions have been studied for some considerable times and it is now established
that hydrogen bonding [9] has a much greater
effect than other types of solvent-solute interactions. The main theories for
shifts due to van der Waals forces between solvent and solute molecules predict
relationships between these shifts and the dielectric properties of the
solvents [4,8]. Berson [2]
has used the mole-bond density of the solvent to correlate the solvent shifts,
observed in determinations of the spectra some aromatic hydrocarbons.
Initially, we studied at about eight solvents; we found that we could obtain
appropriate linear relationships between the absorption frequency for peak of
band at 310 nm (2M NaOH) to 327 nm (ethanol) of benzbhiazoline-2-thione (I) and
functions of the solvent, dielectric constant or refractive index.
The compound
investigated: benzthiazoline-2-thione(I), N-Me derivative of
benzthiazoline-2-thione(II), S-Me derivative of benzthiazoline-2-thione(III),
benzthiazoline-2-thione(IV) and benzthiazoline-2-thione(V) have high extinction
coefficients for the main UV-absorption bands and can therefore be examined in
very dilute solution. For crystalline benzthiazoline-2-thione Bauman [1] have shown that the molecules are arranged in
hydrogen bonded chains among two – fold screw axis with a strong hydrogen bond
to sulfur for which one N-H..... S length is 248 nm. The hydrogen bond in
crystalline benzthiazoline-2-thione is considerably stronger than in
thiopyridyne [10], where the N-H…S length is 326
nm. The effect of hydrogen bonding UV-spectra is because a blue shift with bond
broadening is the chromophore is a hydrogen-bond acceptor and a red shift with
similar broadening of the band when the chromophore is a hydrogen-band donor.
In the oligameric state (I) functions as both a hydrogen-bond acceptor and
donor (except for terminal molecules), there is little next change in the
absorption frequency. Study of the change with solute concentration in calcium
tetrachloride of the relative intensities of the free and hydrogen-bonded N-M
infra-red bands shows that tiazolidine-2-thione, behaves very similarly to
benzthiazoline-2-thione, and thiazoline-2-thione, forms either cyclic species
or very stable linear oligomers of relatively high overage degree of polymerization.
[3]
In
non-hydrogen-bonding solvent such as aliphatic and aromatic hydrocarbons and
their halogen derivatives no shift is observable and the shape of the band
remains unchanged. It is important to establish which species is responsible
for the UV-absorption, but unfortunately it is not passible to use the
infra-red region, where more direct information regarding the extent of
hydrogen bonding can be obtained.
The spectrum of
benzthiazoline-2-thione in solution in diethyl ether is very nearly identical
with that in n-hexane. This is consistent with the view that in this solvent at
the concentration of 10-5M, the benzthiazoline-2-thione molecules
exist almost completely as monomers. Similar can considerably apply to the
spectrum in anizole chromophore (VII):
Both anizole and
diethyl ether have a very low tendency do hydrogen band [5].
It has already been noted that
benzthiazoline-2-thione in the
monomeric and oligomeric states has the same absorption band frequency. This
can be accounted for by postulating that when (I) functions as a hydrogen band
donor and acceptor the next change in the ground-state energy of the
charge-transfer electron is zero. That comparable shifts are produced by donation
and acceptance of a hydrogen band by (I) shown the blue shift at 3 nm in
ethanol and red shift at 3 nm in acetone or methyl ethyl ketone.
A critical test
of these conclusions is provided by the behavior of
3(N)-methyl-benzthiazoline-2-thione in hydroxylic and basic solvents. In
hydroxylic solvents there is a blue shift at 10 nm, comparable with
benzthiozoline-2-thione (I). In dimethyl formamide solution there is no
detectable red shift and in this case none would be expected since there is no
possibility of hydrogen band formation. This shows conclusively that this
solvent shifts are to attributable primarily.
Solute-solvent
hydrogen bonding and not to differences in dielectric constant and refractive
index of the solvents since there is a very wide range.
In glacial acetic acid band in the ranges at 321-332 nm shows
more detail then in n-hexane, although the position of the maximum remains
unaltered so that(I) function as both donor and acceptor at hydrogen bands and
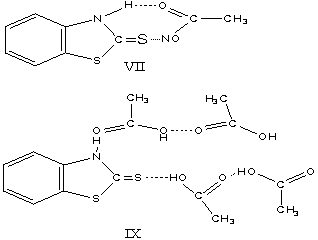
there is no change in absorption frequency. Monocarboxylic acids have been shown to exist as cyclic and open dimers so
that it is possible for benzthiazaline-2-thiane to associate with acetic acid
molecules either as in VIII or IX are formed,
because at the strong hydrogen band donor properties of the hydroxyle
group and acceptor properties of the carbonyl group of the acetic acid .The
spectrum for solutions of benzthiazoline-2-thione in n-butanole closely
resembles, that in glacial acetic acid
indicating that the benzthiazoline-2-thiane molecules function as both
donors and acceptors. This would be consistent with the change in hydrogen bond
strengths referred to in connection with the blue shifts observed in ethanol,
methanol or water.
Results
1. The spectra of benzthiazoline-2-thione (I) N-methyl-benzotiazoline (II) and
s-methyl-benzthiazoline (III) in concentrated sulfuric acid closely resemble
each other.
2. The more basic character of
S-methyl-2mercapto-benzthiazole is shown by its protonation
in dilute hydrochloric acid.
3. The effect of hydrogen bonding to thiazoline-2-thione
in hydroxylic solvents is similar to those benzthiazoline-2-thione.The solvent
shifts for thiazoline-2-thione there are red shifts in ethanol and methanol and
zero shift in water with respect to n-hexane.
References.
1. Bauman R.P.
Absorption spectroscopy / R. P. Bauman // New York, USA, 1997-287 p.
2. Berson J.A. A
qualitative correlation of the spectra of some organic carbonyl compounds
/J.A.Berson//J.Amou. chem. sac. -1993-vol. 115, V 14 pp. 3521-3523
3. Braunde E.A.
Progress in stereo chemistry/ E.A. Braunde, E.S. Wainght, W. Klyne // London,
UK, 1994-549 p.
4. Caldwell D.J.
The Theory of Optical Activity/D.J.Caldwll,H. Eyring//New York, Wiley 1991-244
p.
5. Hollam H.E.
Hydrogen Bonding and solvent effects / H.E. Hollam // Elsevier, Amsterdam,
1993-405 p.
6. Jaffe
H.H. Theory and application of ultraviolet spectroscopy / H. H.
Taffe, M. Orchin // New York, USA, 1992-619 p.
7. Liptay.W.
Excited states in UV-spectroscopy / W. Liptay, E. Lim // New York-London.: Acad. Press., 1994-358 p.
8. Phillips J. P.
spectra-structure correlation / J. P. Phillips // New York-London, 2004-172 p.
9. Robin. N. B.
Higher Excited states of Polyatomic Molecules / M.B.Robin // New York. Academic
press 1994-374 p.
10. Suzuri H.
Electronic absorption spectra and geometry of organic molecules / H. Suzuri //
New York-London, Academic Press, 2007-568 p.
11. West W. Physical
methods of organic chemistry / W.West //
Erd.ed., New York,1999-699 p.