Химия и
химические технологии. 8. Кинетика и катализ
Kalinichenko
O.O., PhD Misnyankin D.O., Dr. Sci. Snizhko L.O.
Ukrainian
state university of chemical engineering, Ukraine
Catalytic burning
of diesel soot
The removal of soot solid
particles consisted of (% mas): C (59-84), H (1-4), O (10-25), N (1-3), S (1-4) and emitted from diesel
engines into the atmosphere is an important environmental problem. Special
filters used nowadays for reducing of soot burn-out temperature as a rule have
the surface catalytic layers. However, the high cost of such systems that
partly made from noble metals, hinders the distribution of such filters. In
this regard, the development of more accessible catalysts for diesel soot
burn-out acquires exceptional importance.
In many catalytic reactions occurring on solid
catalysts, the irregular pore system of the catalyst may negatively influence the selectivity of a
desired intermediate product by slowing down the diffusion of the product molecules in the tortuous pores, thus allowing
their further conversion to undesired by-products. The working conditions of
catalysts to neutralize the extreme vehicle emissions are non-stationary due to
fluctuations of the input parameters of the gas flow, in particular due to
exhaust gas temperature fluctuations that vary from 120-3600C in
cold start and idling up to 50-7000C in steady-state normal regime.
At the same time the catalyst must be capable of withstanding heat up to
900-1100C without loss of activity.
Aluminium oxide is often used as a carrier in catalytic systems thanks to the large surface area and relatively low cost. The structure of the porous anodic alumina films is characterised as a close-packed array of hexagonal cells, each of them containing a central cylindrical pore directed towards the bottom of the oxide film. In order to bring catalytically active species on the alumina carrier, the anodised metals must be immersed in solutions containing the desired metal salts or complexes.
In this work, catalytic layers
were obtained on aluminium (Сu 3.8-4.9, Mg 1.2-1.8, Mn 0.3-0.9, Fe-0.5, Si-0.3, Zn 0.1, Ti,
titanium - 0.1, Al - balance) and titanium (N2- 0.1, C - 0.015, H2 - 0.015, Fe - 0.5, O2 - 0.5, Ti - balance) by anodic spark deposition. The solution of sodium
silicate (2.5 % vol.) was used as electrolyte. The time of electrolysis was 10
min, galvanostatic current density - 400 A/m2, half-periodical positive voltage - 550-600 V. In this technique, the oxide formation takes place by applying an
intense electric discharge on the metal surface acting as anode; the surface
metal melts and at the same time is oxidised, forming characteristic textures
consisting of regular pores. By the appropriate selection of the electrolyte,
active phase precursor, stabiliser and working conditions (potential, current
density, frequency of pulses, time) oxide layers of different thickness (5–50
m) and pore density (1–3×104 pores/cm2) containing the active
component were obtained. The carriers used for anodisation were aluminium and
titanium. Metallic substrates like wires, foils or foils and other more
complicated specimens of aluminium and titanium with a geometric surface of
about 3 cm2 were connected to the anode. Obtained oxide layer was
soaked by the 2 mol/l solutions of Cu(NO3)2⋅3H2O, Ni(NO3)2⋅6H2O,
Mn(NO3)2⋅6H2O, Co(NO3)2⋅6H2O. After every treatment, samples
were dried at 1300C for 5 min and heated at 550°С for 3 hours. Samples with soaked
oxide layers and samples with clean oxide films (for comparison), were covered
with soot in burner flame combustion of diesel fuel (GOST 305-82) for 5 min
each. After infliction, soaked oxide layers together with soot were removed
from the substrates and analysed together with samples of clean soot (Fig. 1).


Fig.1.
Soot deposition on metal plates with catalytic layers
Catalytic
combustion of soot was studied in a dynamic and isothermal regimes using
derivatograph "Paulic-Paulic-Erdey Q-1000" with a heating rate 5°C
per minute. Ignition temperature of soot with a
precision of ± 30°C were estimated from differential thermal analysis (DTA)
curves at the maximum burning rate achieved. Sweep interval of temperatures was
20-500°C. The temperature of the beginning of soot burning was determined by
thermogravimetric (TG) curves, the maximal temperature of the process
determined by the extremum of the differential thermogravimetric (FGD) curves,
with an accuracy of ± 2°C.
Treatment of the thermogravimetric curves was done through every 5
degrees. The conversion degree of reaction were determined as a ratio of the
mass loss of samples (due to oxidation of soot) to mass content of soot
deposited on the sample. Reaction rate was calculated by the equation
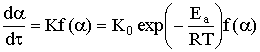 ,
,
were α is conversion degree of the soot
burning, K is constant of reaction rate, К0 is
coefficient, Ea is activation energy, T is temperature, t is
time of burning, f(α) is function which depends on the reaction mechanism.
For
typical topochemical reactions that can be described by the processes of
diffusion, embryos formation, moving of the phase borders and grain growth, the
f(α) can be represented by the equation
![]()
were
m, n, p are numerical constants, correspond to the specific reaction mechanism.
As a result of processing the experimental data, the following
values were found: for activation energy ЕА = 69±5 kJ/mol, for lnK0=3,4±0,5.
Non-catalytic burning
of diesel soot occurs at 6120C, but the soot deposited on Al2O3
oxide burns between 500 and 6400C. So pure
aluminium oxide does not show visible catalytic activity. When soot burns on
the clean cuprum oxide CuO, the burning temperature is 4160C. The
cuprum oxide deposited on Al2O3 decreases the temperature
of soot burning on 60-700C.