Influence of bath
composition on the electrodeposition of MoOx thin film from dimethyl sulfoxide
Akbar Dauletbayb, Washington Braidaa, Mihail Nauryzbaevb, Leila.Kudreeva b
aCenter for Environmental
System, Stevens Institute of Technology, Hoboken, NJ 07030, USA
bChemical and Chemical
Technology Department of al-Farabi Kazakh National University
Abstract
The electrodeposition of
Mo/MoOx from dimethyl sulfoxide has been investigated. The Mo/MoOx
thin films were deposited on copper substrates by the electrochemical
method from dimethyl solfoxide solution. Among the experimental
electrodeposition parameters, only the concentration ratio of additional
substances to molybdate ions was varied to analyze its influence on mechanism
of induced Mo/MoOx deposition. Voltammetry was one of the main
techniques, which was used to examine the voltammetric response, revealing that
Mo/MoOx deposition depended on the nature of the species in
solution. When optimal potential is applied, a colored Mo/MoOx thin
film is formed on the electrode. Chemical ICP-OES analysis was used
to corroborate the almost presence in these films. Intermediate Mo/MoOx
was characterized using scanning electron microscopy (SEM), compositional
analysis was determined with X-ray photoelectron spectroscopy.
1.
Introduction
Molybdenum
is called refractory metal, is widely used for industrial applications such as
heat resistance material because of its high melting point and high strength.
However, molybdenum is often used in simple shapes, such as plate or rod,
because it is hard to be processed. Although the gas phase methods are used
when a complicated shape is demanded, there are some problems in the rate or
the uniformity of deposition. Many investigations have been devoted to the
electrodeposition of with iron group metals [1-4]. However, the mechanism of
molybdenum electrocrystallization is still not elucidated. Indeed this metal
has never been deposited in a pure state from aqueous solutions whereas their
codeposition with iron group metals is possible [5].
Several
hypotheses have been proposed. The most recent investigation mentioned the
possible multistep reduction of some molybdic species leading to a molybdenum
oxide which would be reduced by atomic hydrogen previously adsorbed on the
inducing metal [6].
Results
Influence of citric and boric acid on the
deposition process
Based
in a review of the literature [39-41], boric and citric acid were chosen as
addition species, two kinds of voltammetric responses were observed the citric
and boric acid concentration range. For LiCl + molybdate solutions in the
presence of citric acid 0.001 and 0.01M, boric acid 0.003 and 0.03M, deposition
began slightly after -1V (Fig. 1). The negative scan revealed a double
reduction peak.
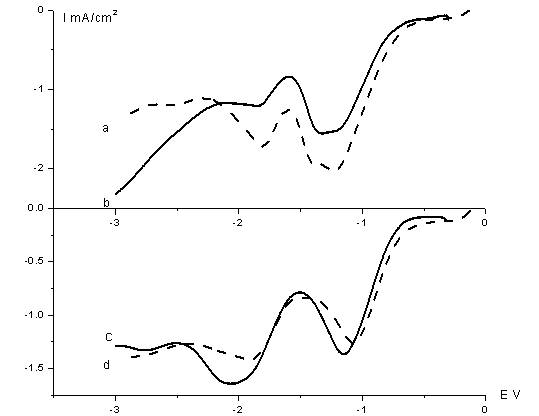
Fig.1
Linear scan voltammogram of 1 M LiCl + 0.01 M (NH4)6Mo7O24
(x M citric + y M boric) acid dimethyl sulfoxide solutions, at 50 mV/s scan
rate. (a) x = 0.001, y = 0;
(b) x = 0.01 y =0; (c) x = 0, y = 0.003; (d) x = 0, y = 0.03.
Table
1.
IPC-OES
analysis of solution obtained after dissolving films in 5 ml of 5% nitric acid.
The bath employed for preparing the films contained 0.01M concentration of
molybdate and variable acid concentrations.
|
E/V C/ mol• L-1 |
0.75 |
-1 |
-1.2 |
-1.35 |
-1.75 |
-1.90 |
-2 |
-2.15 |
|
|
Citric |
0.001 |
0 |
0.0019 |
0.0119 |
0.0254 |
0.0368 |
0.0429 |
0.0469 |
0.0519 |
|
0.01 |
0 |
0.0005 |
0.0089 |
0.0162 |
0.0222 |
0.0314 |
0.0340 |
0.0402 |
|
|
Boric |
0.003 |
0 |
0.0061 |
0.0113 |
0.0246 |
0.0420 |
0.0490 |
0.0593 |
0.0653 |
|
0.03 |
0 |
0.0017 |
0.0093 |
0.0154 |
0.0218 |
0.0315 |
0.0372 |
0.0499 |
|
Moreover, the
charge involved in the first and second reduction processes were increased by
increasing the citric acid concentration (Fig. 1,
curve a, b), but when the presence of boric acid revealed decreasing on the
contrary (Fig. 1, curve c, d). Can be
supposed, citric and boric acid was more helpful for decomposition of DMSO,
also prevented deposition of molybdenum compounds. Because both of these acids
produce more protons. Table 2 corroborated this supposition. The films were deposited to sue recurrent
potential pluses methods different constant potentials and time (20 minutes). Furthermore,
ICP-OES analysis revealed that the molybdenum compound decreased greatly when
the presence of acids in the bath solution.
References
[1] E.
Chassaing and K.Vu Quang, and surf. treatment 2 (1973/74) 65.
[2] K.Vu
Quang, E. Chassaing, F. Bourelier and J. Montuelle, Corrosion T.P.F 19 (1971)
137.
[3] M.
Cherkaoui, E. Chassaing and K.Vu Quang, Plaring and Surf. Finishing, Oct.
(1988) 50.
[4] C. C.
Nee, W. Kim and R. Weil, J. Electrochem. Soc. 135 (1988) 1100.
[5] A.
Brenner in ‘Electrodeposition of Alloys’ Academic Press, New York an London
(1963) Vol. 2, p.429.
[6] H.
Fukushima, T. Akiyama, S. Akagi and K. Higashi, Trans. Japan Inst. Metals 20
(1979) 358.
[7]Elvira
Go´mez, Eva Pellicer, Elisa Valle´s, Journal of Electroanalytical
Chemistry 556 (2003) 137_/145
[8] Elvira Go´mez, Eva Pellicer, Elisa Valle´s, Journal of Applied
Electrochemistry 33: 245–252,
2003. 245.
[9] Qiaoying Zhou,
Hongliang Ge, Guoying Wei, and Qiong WuJournal of University of Science and
Technology Beijing Vol 15, ¹ 5, 2008,
611.