Ýêîëîãèÿ / 6. Ýêîëîãè÷åñêèé ìîíèòîðèíã
PhD, ing. Siedlecka E.
Management
of by-products from flotation waste utilization technology
Abstract. The primary direction
of environment protection is waste source reduction by non- waste technology,
eventually by recycling. Waste deposition should be avoided. The amount of
waste is ecological and geotechnical problem. In the paper flotation wastes
from Zn-Pb ore enrichment that have been damped for decades and waste acids are
described. The main component of flotation wastes – dolomite (~75%) that have
alkaline properties can be used to waste acids neutralization. Metals contained
in flotation wastes and in waste accumulator electrolyte are the same. This
phenomenon suggest that process of neutralization is favorable because does not
increase the processes of solution purification. In addition, it is the base of
profitability the mineral fertilizer receiving that perform the standards.
Technological idea of utilization the both accumulator electrolyte and Zn-Pb
flotation waste was introduced. Possibilities of by-products management as
inorganic pigments were defined. In the paper chemical, thermal and sieve
analysis were presented.
1. Introduction. Flotation tailings
constitute the largest mass of waste from the Zn-Pb mining sector. They are
damped in the landfills formed by the gradual raising of embankments and
replenishment. Landfills used by a dozen and sometimes even decades attain
considerable size and height (table 1) [1].
Economic
utilization of flotation wastes is negligible at the present day, although
studies on their usefulness in various sectors of the economy have been carried
out since the seventies. Residues of metals made that the flotation waste dumps
in recent years have been qualified as a unbalanced ore resources. It improved
the economic indicators by artificial means and help the companies to avoid
fees and penalties for the use of the environment.
Table 1. Characteristic of flotation wastes dumps in Poland [1]
|
Waste dumps owner |
Location |
Area, ha |
Height, m |
Waste quantity, mln Mg |
Annual increase, mln Mg |
Final waste quantity, mln Mg |
|
ZGH Bolesław |
Bukowno |
108 |
20 |
43,6 |
about 1,6 |
67,6 |
|
ZGH Trzebionka |
Chrzanów |
64 |
28* |
18,5 |
about 1,6 |
32,9 |
|
ZGH Orzeł
Biały |
Bytom |
110 |
about 15 |
18,9 |
0 |
18,9 |
* target
height 35m
Enormous
estimated resources of zinc, lead, iron and other metals in the flotation wastes
could suggest that these wastes can be used as the secondary raw material
deposits (table 2) [2].
Table 2. Estimated resources of metals in the inactive landfills of ZGH
Boleslaw in Poland [2]
|
Component |
Resources, Mg |
|
Zn Pb Fe S Cu Cd Ag As Tl Ni Co Cr Ba Sr ZnO PbO Zn:Pb |
250 479,4 117 710,0 1 714 533,0 1 686447,7 4 846,7 1 967,2 76,5 13 105,7 664,9 549,9 132,0 245,8 3 263,4 1 801,6 133 739,6 83 428,5 2:1 |
Oxidation
of zinc and lead in flotation wastes is 90%. This is a result of selectivity of
the flotation process, in which the concentrates are sulphides of Zn and Pb.
The mineral oxides of these metals are residues. They are the main form of
mineral occurring in the flotation wastes.
Low
content of zinc and lead and the presence of these metals in combination with
oxygen suggests that the current technologies will not be able to receive good
quality of flotation concentrates. Therefore, it seems unreasonable that they
may be raw material deposits.
Flotation
wastes were attempted to use in various ways. One solution was managing them as
a mineral filler in the paper, rubber and plastics [1,3]. Applying the wastes
in production of building materials and in agriculture for de-acidification of
soils was assumed. Too high content of heavy metals in the flotation wastes was
an obstacle in manage them. A possibility of depositing flotation wastes under
the ground as a stowage was considered too. The condition of this solution,
however, is that the flotation waste which is a substitute for sand stowage
would be never in the saturated zone [4,5]. This would cause a pollution of the
underground, especially mine water.
The
basic criterion for the use of flotation wastes is to develop technologies for
the separation of heavy metals and utilization of by-products in other industrial
technologies. The technology of waste mineral acids utilization by flotation
wastes presented in the paper is characterized by using two kinds of wastes in
one process. This is the basis of its profitability.
2. Materials and methods. This work presents research on possibilities of
by-products management from waste
accumulator electrolyte utilization technology. For this purpose, thermal,
chemical and phase analysis of products were performed. Thermogravimetric
analysis was carried out with a SETARAM LabsysTM derivatograph in
argon atmosphere. The concentrations of metals in the filtrates were determined
by ICP method. The phase composition of by–products (inorganic pigments) was
determined by a Bruker D8 ADVANCE X-ray difractometer using Cu Ka radiation with a
tube voltage of 40 kV, a tube current of 30 mA and scanned from 2°-60°.
3. Characteristic of flotation waste. The flotation wastes from the zinc and lead industry
from the landfills in Bytom district were used. Chemical analysis (table 3),
grain composition (table 4) and phase analysis (figure1) showed that the basic
metals in the flotation waste are calcium, magnesium, iron, zinc, lead, small
amounts of manganese, aluminum, arsenic, silicon, cadmium, copper and cobalt. Metals
occur mainly in the form of carbonates, oxides and sulphides [6, 7].
Table 3. Chemical
composition of flotation waste [6]
|
Metal |
Ca |
Mg |
Fe |
Zn |
Pb |
Mn |
Al |
As |
Si |
Cd |
Cu |
|
% |
20,0 |
9,0 |
6,0 |
1,52 |
0,44 |
0,43 |
0,26 |
0,12 |
0,022 |
0,0098 |
0,0049 |
Table 4. Grain
composition of flotation waste [6]
|
Size mm |
Content % |
Total % |
|
>2 |
0,10 |
0,10 |
|
2-1 |
0,30 |
0,40 |
|
1-0,63 |
0,53 |
0,93 |
|
0,63-0,2 |
4,80 |
5,73 |
|
0,2-0,09 |
13,27 |
19,00 |
|
0,09-0,06 |
15,57 |
34,57 |
|
0,06-0,02 |
31,92 |
66,49 |
|
0,02-0,01 |
9,80 |
76,29 |
|
<0,01 |
23,71 |
100,00 |
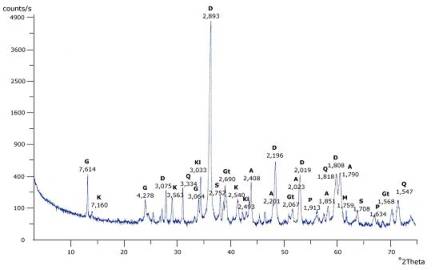
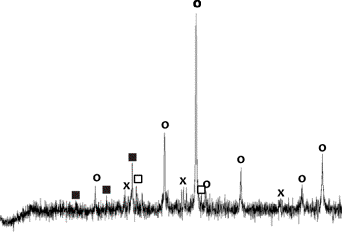
Figure 1. The phase composition of flotation
waste: (D - dolomite, A - ferruginous dolomite, K-calcite, Kl – kaolinite, Q -
quartz, P- pyrite, S - sphalerite, Gt - goethite, G - gypsum, M - marcasite)
[7]
Thermogravimetric
analysis of flotation waste (figure 2) determines the content of main component
– dolomite at 70%. The high content of alkaline components assures high
efficiency of neutralization process. Metals occurs in flotation waste are the
same as in waste accumulator electrolyte (table 5). This increases the number
of processes used to purify the solution [6, 7].
Gypsum=4,4% Dolomite=69,9% Calcite=0,748%
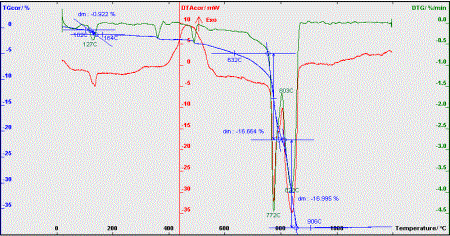
Figure 2. Thermogravimetric analysis of
flotation waste (25-10000C, 100C/min, argon) [7]
Table 5. Chemical composition of waste accumulator electrolyte [7]
|
Metal |
Fe |
Zn |
Cu |
Cd |
As |
Pb |
Mn |
Sn |
Co |
|
Concentration, mg/dm3 |
8,3×102 |
5,0×102 |
69 |
18 |
4,5 |
4,0 |
2,5 |
2 |
0,58 |
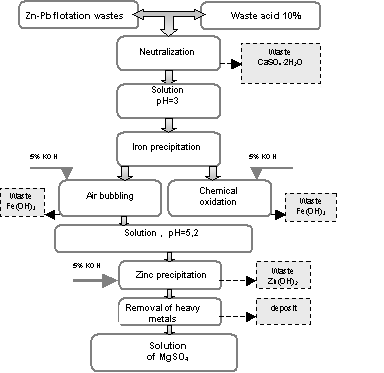
4. Characteristic of technology. Technological diagram of waste accumulator electrolyte
utilization is shown in figure 3. A final product of this technology is a solution of
magnesium sulphate.
The
process of neutralization determines the quantity of processed liquid, metal
concentration in the eluat and the quality of post-neutralization slime. Metal
concentrations (Mg, Zn, Cd, Pb, Fe) in the post-neutralization solution are
presented in table 6.
Efficiency
of metals in the post-neutralization solution is high and depends on the time
of leaching (table 7).
|
|
|
Figure 3. Technological schematic diagram of
magnesium sulphate recovery from flotation wastes and waste mineral acid
utilization process [6] |
Table 6. Metal concentrations in the post-neutralization
|
pH |
Mg mg/dm3 |
Zn mg/dm3 |
Pb mg/dm3 |
Cd mg/dm3 |
Fe mg/dm3 |
|
1 2 3 4 |
6,3×103 12,6∙103 2,1∙104 2,2∙104 |
1248 2482 3556 3461 |
2,80 3,60 3,50 7,50 |
7,46 15,5 22,0 21,07 |
2560 4677 6400 6145 |
Table 7. Dependence
of efficiency of metals in the post-neutralization solution on the time of
leaching
|
Time of leaching, min |
Mg mg/dm3 |
Ca mg/dm3 |
Fe mg/dm3 |
Pb mg/dm3 |
Cd mg/dm3 |
Zn mg/dm3 |
|
0,5 |
9,1×103 |
5,0×102 |
3,6×103 |
2,4 |
7,3 |
1,8×103 |
|
1 |
1,0×104 |
5,0×102 |
4,0×103 |
2,6 |
7,4 |
1,8×103 |
|
1,5 |
1,1×104 |
5,1×102 |
4,0×103 |
2,6 |
7,8 |
1,9×103 |
|
2 |
1,7×104 |
5,2×102 |
4,8×103 |
2,8 |
9.5 |
2,4×103 |
|
5 |
1,7×104 |
5,1×102 |
4,85×103 |
3,5 |
17 |
2,4×103 |
|
10 |
1,7×104 |
5,1×102 |
4,85×103 |
3,5 |
19 |
2,4×103 |
|
15 |
1,8×104 |
5,1×102 |
4,85×103 |
3,5 |
20 |
2,45×103 |
|
30 |
1,8×104 |
5,2×102 |
4,9×103 |
3,5 |
21 |
2,9×103 |
|
60 |
1,9×104 |
5,2×102 |
5,5×103 |
3,5 |
23 |
3,3×103 |
|
120 |
2,0×104 |
5,3×102 |
6,2×103 |
3,5 |
23 |
3,4×103 |
|
180 |
2,1×104 |
5,2×102 |
6,4×103 |
3,5 |
23 |
3,6×103 |
|
240 |
2,0×104 |
5,2×102 |
6,5×103 |
3,5 |
23 |
3,6×103 |
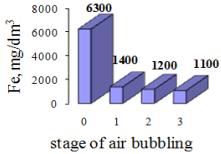
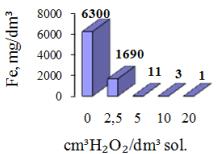
The
post-neutralization solution is characterized by high content of iron and zinc,
which should be separated because of the environment protection. The process of
iron precipitation from the solution was carried out by alkalization to pH 5.2
with 5% of KOH. Additionally, the bubbling air or chemical oxidation with 30% H2O2
was applied. The concentrations of the metals in the solution after iron
precipitation are shown in the figures 4 and 5.
|
|
|
|
Figure 4. Concentration of Fe after air bubbling and alkalization |
Figure 5. Concentration of Fe after chemical oxidation and alkalization |
Precipitation
of zinc by alkalization to pH 8.2 and the final stage of purification –
cementation, led to a magnesium sulphate solution. Composition of this solution
was compared with the maximal permissible values for mineral fertilizers (table
8).
Table 8. Concentration of metals in MgSO4
|
Metal |
Concentration in MgSO4, mg/kg s.m. |
Maximal permissible values for mineral fertilizer, mg/kg s.m. |
|
|
A* |
B** |
||
|
Cu |
0,09 |
0,09 |
400 |
|
Fe |
0,8 |
1,12 |
- |
|
Pb |
<0,11 |
<0,11 |
140 |
|
Zn |
0,6 |
3,03 |
1500 |
|
Mn |
1,7 |
2,58 |
- |
|
Cd |
0,11 |
0,11 |
50 |
|
Co |
<0,11 |
<0,11 |
- |
|
As |
<0,11 |
<0,11 |
50 |
*post-cementation
solution, pH 5.8
**post-cementation
solution, pH 7.0
Solution
of MgSO4 fulfills the requirements for mineral fertilizers. It can
be applied in liquid form or after the process of crystallization to solid
form.
5. Management of by-products. The proposed technological diagram (figure 3) assumes
by-products production. Their management in the industrial technologies is the
basis of waste-free and environmental friendly technology .
Gypsum= 92%
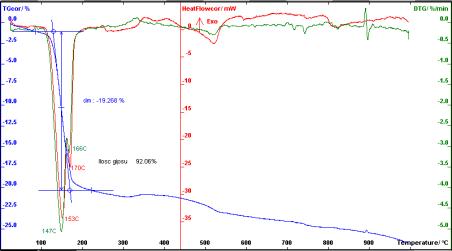
Figure 6. Thermogravimetric analysis of the post-neutralization
slime
One of
the by-products is post-neutralization slime. The main compound of the slime is
CaSO4∙2H2O. It should be noted that the slime with
content of gypsum over 91% can be used in the cement industry as an additive
for slowing down the cement setting. The quality of the slime fulfills these
requirements. Thermogravimetric analysis of the post-neutralization slime
(figure 6) determines the main compound of the slime – gypsum at a rate of
91-92%.
Analysis
of the results of purification of the solution from zinc at pH 8.2 indicates a
high degree of zinc removal of 99,97%. However, co-precipitation of magnesium occurs
at this pH value. This is a negative phenomenon for the whole technology. The
result of zinc precipitation from the solution is shown in table 9.
The
efficiency of precipitated zinc increases with the change of zinc precipitation
conditions by alkalization from pH 7.0 to pH 8.2. The highest zinc efficiency (99,94%)
was obtained at pH 8.2. At highest values of pH the co-precipitated effect was
observed. As a result, a decrease in magnesium concentration was noticeable.
Table 9. Concentration of metals in the solution on the pH values
|
metal |
Mg mg/dm3 |
As mg/dm3 |
Cu mg/dm3 |
Fe mg/dm3 |
Pb mg/dm3 |
Cd mg/dm3 |
Zn mg/dm3 |
Mn mg/dm3 |
|
pH 5.2 |
2,0·104 |
<0,01 |
0,082 |
3,0 |
0,2 |
19 |
2400 |
810 |
|
pH 7.0 |
2,0·104 |
<0,01 |
0,08 |
3,0 |
0,2 |
19 |
1176 |
810 |
|
pH 7.2 |
2,0·104 |
<0,01 |
0,08 |
3,0 |
0,2 |
19 |
320 |
810 |
|
pH 7.4 |
2,0·104 |
<0,01 |
0,08 |
3,0 |
0,2 |
19 |
300 |
810 |
|
pH 7.6 |
2,0·104 |
<0,01 |
0,07 |
2,5 |
0,2 |
19 |
136,8 |
810 |
|
pH 7.8 |
1,9·104 |
<0,01 |
0,07 |
2,0 |
0,015 |
18 |
68 |
700 |
|
pH 8.0 |
1,9·104 |
<0,01 |
0,06 |
0,5 |
0,015 |
18 |
1,8 |
600 |
|
pH 8.2 |
1,8·104 |
<0,01 |
0,04 |
0,5 |
0,01 |
18 |
1,4 |
520 |
The results of the study indicate that the
two-stage alkalization process is required (first step to pH 7.6, second step
to pH 8.0). The deposit of Zn(OH)2 from the first step (precipitated
from the solution at pH 7.6) could be a product of economic importance. The deposit
from the second step (precipitated at pH 8.0) after roasting can be used in the
method of hydrometallurgical zinc production. The content of ZnO in Zn(OH)2
from the first step is presented in figure 7.
Zn(OH)2=
99,12%
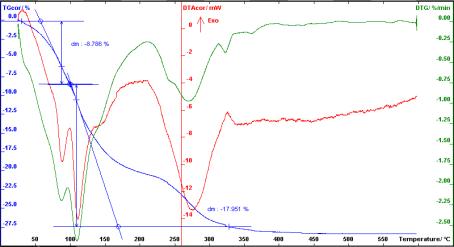
Figure 7. Thermogravimetric analysis of
deposit of Zn(OH)2
Practically,
too complex technologies should be avoided in the industry, therefore the two-stage
alkalization process is not a good solution.
Selectivity
of the iron precipitation is not effective. The deposit of Fe(OH)3
with a large content of zinc (~ 30%) as well as complications in obtaining pure
concentrates of Zn(OH)2 decided that both can be used together in an
inorganic pigment production, mainly a brown ferric. In order to obtain the
brown ferric the mixture of the hydroxides has to be burned. The brown ferric consist of ferric oxide,
zinc oxide and magnesium or manganese oxide, for example: 33,7% – ZnO i 66,3% Fe2O3
[8].
Chemical
analysis and mass fraction calculation showed that the percentage of compounds
ought to be as follows: 32,2% ZnO and 67,8% Fe2O3. For
that purpose, it is necessary to add Zn(OH)2. In this way we can
obtain the proper mixture mass to burning.
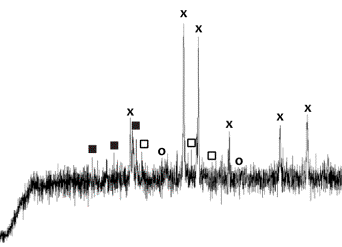
Depending on the method of
neutralization and thermal conditions iron pigments in shades of red, black or
bronze are obtained. The phase composition of the pigments was determined by
means of X-ray analysis (figure 8). The main component of the samples of
pigments is hematite or magnetite.
The use of two waste to the
inorganic pigment production appears to be a logical and most beneficial way of
their management. Currently, research in the semi-technical scale is carried
out in association with obtaining larger amounts of mixture and suitable
certificates.
|
sample number 1 o- FeSO4 n - Fe2(SO4)3 X - hematite O - magnetite |
||||
|
sample number 2 o- FeSO4 n - Fe2(SO4)3 X - hematite O - magnetite |
||||
|
Figure 8. XRD analysis of the pigments |
6. Conclusions. On the basis of the research conducted, the following
conclusions were drawn:
·
presented technology assumes maximization of the substrates (accumulator
electrolyte and flotation waste) what is the basis of its profitability,
·
the main product of technology is a solution of MgSO4,which fulfills
the requirements for mineral fertilizers,
·
there is possibility of using a post-neutralization slime with content
of gypsum over 91% in the cement industry as an additive for slowing down the cement
setting,
·
selectivity of the of Fe(OH)3 and of Zn(OH)2
precipitation is not effective, what determines that both of them can be used
together in an inorganic pigment production (mainly a brown ferric),
·
it is necessary to add Zn(OH)2 or ZnO to the mixture of precipitated
hydroxides in order to obtain the proper composition of the mixture to be
burnt,
·
the method of iron and zinc precipitation as well as the thermal
conditions of burning the mixtures determine the colors and phase composition
of iron pigments,
·
management of by-products from presented technology makes its wasteless
and environmental friendly.
Rreferences
1.
Girczys J.K.,
Sobik-Szołtysek J., Odpady przemysłu cynkowo - ołowiowego,
Wydawnictwo Politechniki Częstochowskiej, Częstochowa 2002.
2.
Ney R., Surowce
mineralne Polski, Surowce metaliczne cynk i ołów, Wydawnictwo
CPPGSMiE, PAN, Kraków 1997.
3.
Sztaba K.,
Kuczyńska I., Sanak-Rydlewska S., Ociepa Z., Utylizacja odpadów cynkowo ołowiowych, Rudy i Metale
Nieżelazne, 41, 3, 154-158, 1996.
6.
Siedlecka E.,
Wykorzystanie odpadów z flotacji blendy cynkowej w utylizacji kwasu
siarkowego, Ochrona środowiska i zasobów naturalnych, nr 33, Warszawa,
2007.
7.
Siedlecka E.,
Doniecki T., Ocena możliwości zagospodarowania odpadowego
wodorotlenku cynku z technologii utylizacji elektrolitu akumulatorowego, Zeszyty Naukowe Politechniki Rzeszowskiej,
Budownictwo i Inżynieria Środowiska 2009, nr 268, s. 87-101.
8.
Kula M.,
Sobolewski W., Tlenki żelaza – ważne pigmenty dla przemysłu
budowlanego, Tworzywa Sztuczne i Chemia, Nr 1, 2005.