Dukhinova M.S., Lipina T.V., PhD,
Pogodina L.S., PhD
Lomonosov Moscow State University, Russia
Analysis of age-dependent changes in myocardium of Japanese quail
BACKGROUND
Cardiovascular
diseases are the leading reason of human death all over the world (2, 4). Heart
aging – one of the main factors in their developing – is rather well studied
only in mammals. Birds are often used in embryonic development research but
there is lack of information about their aging (1). Mammalian and avian hearts
have similar anatomy structure but avian heart is bigger due to body weight (3).
It is an obvious advantage of the model. Moreover, avian cardiomyocytes
constantly undergo the mechanical stress because of their faster heart rate (7).
Japanese quails, Coturnix japonica,
are also characterized by natural accelerated aging and can become one of the
promising model objects in gerontology.
MATERIALS AND METHODS
Japanese
quails (males and females) 9-12 (young) and 48-52 (old) weeks old (from Emanuel
Institute of Biochemical Physics RAS) were used in our research. The left-ventricle
myocardium’s tissue samples were prepared for light and transmission electron
microscopy studying. The cardiomyocyte size, number of blood capillaries per
one cardiomyocyte and apoptotic death level (by Tunel method) were analyzed at
thick sections. Volume of myofibrils, mitochondria and lipid drops, number of
long mitochondria and intermitochondrial contacts (criteria of functional heart
muscle overload, (5)) were measured using electron-microscopy method. The data
were analyzed by Statistica 5.5 (Mann-Whitney nonparametric U-test, ð˂0,05).
RESULTS
AND DISCUSSION
Light-microscopic
analysis revealed that Japanese quail’s cardiomyocytes are long narrow cells
about 3 µm in diameter with thin sheets of connective tissue between them. The
number of blood capillaries per one cardiomyocyte is 0,86. These parameters
were at the same level in birds of both analyzed age groups.
Although
no evidence of hypertrophic changes was found during light-microscopic
research, the level of apoptotic cell death is increasing in two times during
the studied period of quail life. The influence of cardiomyocytes’ shortage can
be already seen on electron-microscopy level.
Electron-microscopic
analysis revealed the nucleus of Japanese quail’s cardiomyocyte is situated in
center and has elongate form and invaginated borders. The main part of
cytoplasm (up to 70%) is occupied by myofibrils that organized in parallel
rows. In old quails’ cardiomyocytes this type of myofibrils’ organization is
sometimes damaged. From 9 to 52 weeks of life myofibrils volume is significantly
decreased. However, the particular zones where contractile apparatus is
hypertrophied can be seen in cells of old birds.

Picture 1. Cardiomyocyte of 9 weeks old
Japanese quail
Mitochondria
are localized between myofibrilar rows and in perinuclear region; their volume
is 30% of cytoplasm at the average. The mitochondria’s volume is significantly increased
in the cells of 52 weeks old birds. So, the mitochondria/myofibrils ratio is
shifted to mitochondria in old birds’ heart, comparing with younger ones.
Due
to localization, mitochondria are divided into two groups, or populations:
interfibrilar and perinuclear mitochondria. These organells differ by its size
and shape. Perinuclear mitochondria are mostly not very big, round- or
oval-shaped organells. In interfibrilar zone long and large ones can be seen,
but not very often. We account mitochondrion as long if it is more than 2 µm in
length. It can be clearly seen that large mitochondria are formed by fusion of
the usual organells.


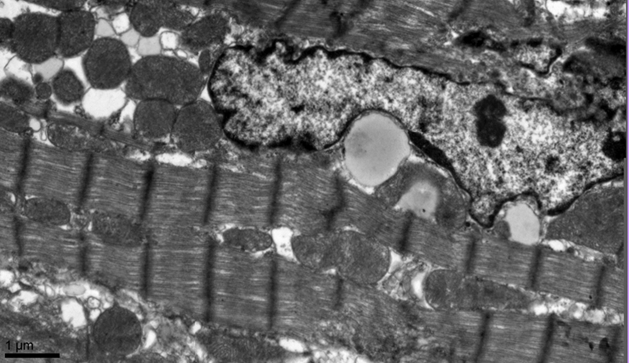
Picture 2. Cardiomyocyte of 52 weeks
old Japanese quail
The
senescence-connected structural and functional alterations of mitochondria can
be seen on electron-microscopy level. First of all, the number density of long
mitochondria increased by 60% from 9 to 52 weeks age. The longest
mitochondrion’s profile measured in young cardiomyocytes is 3,3 µm and runs to
6,6 µm in the old ones.
Age-related changes have different
influence on number of intermitochondrial contacts in interfibrilar and
perinuclear zones. No changes are marked in the interfibrilar population of
mitochondria. At the same time in the perinuclear zone we can see the increase
of intermitochondrial contacts’ number from 9 to 12 weeks and decrease of its
quality to 52 weeks of age back to the level of 9 week-old group.
Both
in interfibrilar and perinuclear zones lipid drops and lipofuscin granules can
be seen.
Lipofuscin
granules can be detected in cardiomyocytes of young and old quails, but its
quality significantly increases in old birds. This
fact corresponds well to data mentioned earlier for aging in humans (8) and in
rodents (6). Lipofuscin granules in quail’s
cardiomyocytes are heterogeneous, electron-dense structures of different shape
and size.
The
volume of lipid drops increases on 40% during studied period of life. This
fact, together with mentioned above mitochondria changes, are likely to indicate
alterations in cell energy metabolism during quail life.
So,
we found that quail myocardium of 48-52 weeks old birds (old), in comparison
with 9-12 weeks old, have several differences: increased number of apoptotic
cells; increased mitochondria/myofibril ratio, increased number of long
mitochondria, higher level of lipid drops volume and lipofuscin granules
quality in cardiomyocytes of old heart. All mentioned changes were the same for
males and females - we found no sexual distinctions in aging process in quail
myocardium.
ACKNOWLEDGMENTS
We
thank Dr. Pavel P. Zak and employees of Laboratory of Photo-Chemical Bases of
Reception, Emanuel Institute of Biochemical Physics RAS, for provided research
material and assistance in experiments realization.
REFERENCES
1. Henk P. J. Buermans, Bram van Wijk, Margriet A. Hulsker, Niels C. H. Smit,
Johan T. den Dunnen, Gertjan B. van Ommen, Antoon F. Moorman, Maurice J. van
den Hoff, Peter A. C. ’t Hoen. Comprehensive Gene-Expression Survey Identifies
Wif1 as a Modulator of Cardiomyocyte Differentiation. PLoS ONE |
www.plosone.org December 2010 | Volume 5 | Issue 12 | e15504
2. Dao-Fu Dai and Peter S. Rabinovitch. Cardiac Aging in Mice and Humans:
the Role of Mitochondrial Oxidative Stress. Trends Cardiovasc Med. 2009 October;
19(7): 213–220. doi:10.1016/j.tcm.2009.12.004.
3. Rumyantsev P.P. Cardiomyocytes in reproduction, differentiation and
regeneration. “Nauka”, 1982.
4. H. Shih, BA, B. Lee, BA, R. J. Lee, MD, PhD, and A. J. Boyle, MBBS, PhD.
The Aging Heart and Post-Infarction Left Ventricular Remodeling. J Am Coll
Cardiol. 2010 December 28; 57(1): 9–17. doi:10.1016/j.jacc.2010.08.623.
5.
Shornikova M.V. Intermitochondrial
contacts in cardiomyocyte mitochondriom in norm, under physiological overload
and in pathology. Onthogenesis. – 2000. - ¹ 6. – Pp. 470-475
6.
J. N. Skepper and V. Navaratnam.
Lipofuscin formation in the myocardium of juvenile golden hamsters: an
ultrastructural study including staining for acid phosphatase. J. Anat. (1987), 150,
pp. 1554167
7. David B. Slautterback, Ph.D. Mitochondria in cardiac muscle cells of the
canary and some other birds. The Journal of Cell Biology, Volume 4, 1965.
8. Strehler BL, Mark DD, Mildvan AS. GEE MV: Rate and magnitude of age
pigment accumulation in the human myocardium. J Gerontol. 1959 Oct; 14:430-9.