INFLUENCE OF
QUERCETIN/IRON (II) COMPLEX ON PHASE TRANSITIONS OF PHOSPHATIDYLCHOLINE OR
PHOSPHATIDYLETHANOLAMINE CONTAINING LIPOSOMES
Elena A. Yagolnik a, Yury S.
Tarahovsky b,c,*, Svetlana M. Kuznetsovab, Eugeny N.
Muzafarov a,d, Yuri A. Kim e
aTula State
University, Tula, 300000, Russia
bInstitute of
Theoretical and Experimental Biophysics, RAS, Pushchino, Moscow Region, 142290,
Russia
cResearch-Educational
Center "Bionanophysics," Moscow Institute of Physics and Technology,
141700, Dolgoprudniy, Russia
dInstitute of Basic
Biological Problems, RAS, Pushchino, Moscow Region, 142290, Russia
eInstitute of Cell
Biophysics, RAS, Pushchino, Moscow Region, 142290, Russia
ABSTRACT
We
studied influence of quercetin/iron (II) complexes on phase transitions of the phospholipid bilayer of
liposomes. Differential scanning calorimetry (DSC) of liposomes prepared from
dimiristoyl-phosphatidylcholine (DMPC) and
palmitoyl-oleoyl-phosphatidylethanolamine (POPE) demonstrated that
quercetin/iron complexes cannot interact with liposomes because, as it was
detected by photon-correlation spectroscopy, they produce nano- and
micro-particles insoluble in water. However if quercetin was added to liposomes
followed by iron, the quercetin/iron complex interacted with the phospholipid
bilayer and influenced lipid phase transitions. The most pronounced changes
were observed in the transition from the bilayer to the hexagonal HII
phase of POPE liposomes treated by quercetin followed by iron. DSC detected a
considerable increase in the transition width and a shift of the transition
maximum towards higher temperatures. The revealed influence of flavonoid/iron
complexes on lipid phase transitions should be considered in designing
liposomal vehicles for flavonoids and drug delivery.
Flavonoids
attract attention because they can influence the human health. These natural compounds
are regarded not only as potent antioxidants, but also as important biological
regulators essential for prevention of various diseases including
cardiovascular, autoimmune, inflammatory, endocrine, neurodegenerative, and
cancer [1]. Because of hydrophobicity they
are preferably interact with biological membranes [2]. Here we studied
the influence of quercetin on two most important phospholipids of cell membranes
phosphatidylcholine and phosphatidylethanolamine. Phosphatidylcholine tends to
produce stable bilayer in a wide range of temperatures.
Phosphatidylethanolamine is able to produce bilayer structures only in the
vicinity of the lipid melting point known as the main phase transition (Tm).
At higher temperature phosphatidylethanolamine is subjected to a transition
from the bilayer to the hexagonal HII phase. The transition
temperature depends the on hydrocarbon
chains composition [3].
Phosphatidylethanolamine isolated from mammalian cells may produce hexagonal HII
phase at a physiological temperature. It is supposed that in a living cell the
ratio of lipids producing bilayer and nonbilayer structures is homeostatically
regulated to optimize conditions for membrane proteins functioning. A
disbalance of lipid phase properties could be a reason of numerous diseases [4].
The ability of
flavonoid-iron complexes to interact with the bilayer was demonstrated by DSC.
As follows from DSC thermograms, iron cations in concentrations 10-6
M, which is close to the iron concentration in human blood, had a little effect
on the DMPC melting (Fig.1), while quercetin-iron complex influenced the lipid
melting weaker than free quercetin. The effect could be observed only when
quercetin was added to liposomes first and about 30 minutes later the iron
cations were added. In the cases when the compounds were added in the reverse
order, or when the preliminary prepared mixture of quercetin and iron were
added to liposomes we did not see any influence of the complex on the lipid
melting.
A similar change of
Tm transition was observed after sequential additions of quercetin
and 30 min later of iron to POPE liposomes (Fig.2). The influence of
flavonoid-iron complexes on Th transition of POPE at 69°C was much
stronger than
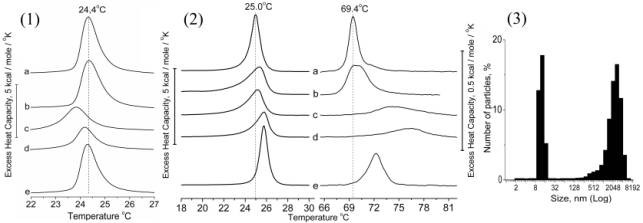
Fig. 1 and 2.
Influence of quercetin and quercetin/iron complexes on DSC thermogram of: (1) –
DMPC liposomes . Concentration of lipid was 2,95·10-4 Ì (0,2 mg/ml), quercetin – 4·10-5 Ì, FeSO4
– 4·10-6 M; (2) – POPE liposomes. Concentration of lipid was 1,95·10-3
M (2 mg/ml), quercetin – 4·10-4 Ì, FeSO4 – 4·10-5 M. The thermograms presented here
are of untreated liposomes (a); liposomes treated by quercetin (b); treated by
iron (c); treated by quercetin followed by iron added 30 min later (d); treated
by preliminary produced quercetin/iron complex (e). For DSC we used DASM-4 (IBP
RAS, Pushchino, Russia).
Fig. 3. Photon-correlation spectroscopy analyses of
particle size present in mixture of quercetin and FeSO4 in
concentration used for DSC. The particle size distribution was analised on N4 PLUS Submicron
Particle Size Analyzer (Beckman Coulter, USA).
that on Tm
transition described above. In the presence of quercetin-iron complexes the
maximum of the transition was shifted by about 5 degrees to higher temperatures
while the width of the transition increased and the height decreased. These
changes were more pronounced after the sequential addition of quercetin and
iron. Addition of preliminarily prepared complexes of quercetin and iron had
much lower effect on the lipid transition. This effect could be explained by
low solubility of quercetin-iron complex in water and formation of insoluble
particles of 10 – 15 nm and of 1 – 5 μm detected by photon-correlation
spectroscopy (Fig.3).
Phosphatidylcholine
and phosphatidylethanolamine are often used for preparation of liposomes
applicable for drugs and genes delivery. The delivery efficiency depends on the
phase transitions of lipids [5]. The ability of
flavonoid-metal complexes to influence the phase behavior of
phosphatidylethanolamine could be especially important in liposomes containing
this lipid, for example those used in gene therapy [6]. Liposomes loaded with
flavonoids may considerably increase the flavonoids concentration in blood and
facilitate their bioavailability [7]. Lipophilic complexes
of flavonoids with iron could be useful for development of liposomal vehicles
for drug and gene delivery.
Acknowledgment
We thank CCU MIPT and REC
"Nanotechnology" of MIPT for
the equipment and chemicals used in this work. Financial support from the
ONEXIM group and the Skolkovo Foundation and the Ministry of Education and
Science of the Russian Federation, agreement 14.B37.21.1515 is gratefully
acknowledged.
Reference
List
[1] S.
Nishiumi, S. Miyamoto, K. Kawabata, K. Ohnishi, R. Mukai, A. Murakami, H.
Ashida, and J. Terao, Front Biosci.
(Schol. Ed), 3 (2011) 1332-1362.
[2] A.B.
Hendrich, Acta Pharmacol. Sin., 27
(2006) 27-40.
[3] M.
Rappolt, A. Hodzic, B. Sartori, M. Ollivon, and P. Laggner, Chem. Phys. Lipids, 154 (2008) 46-55.
[4] R.
Phillips, T. Ursell, P. Wiggins, and P. Sens,
Nature, 459 (2009) 379-385.
[5] Y.S.
Tarahovsky, Biochemistry (Mosc. ), 75
(2010) 811-824.
[6] Y.S.
Tarahovsky, Biochemistry (Mosc. ), 74
(2009) 1293-1304.
[7] Z.P. Chen, J. Sun, H.X. Chen, Y.Y. Xiao, D. Liu, J. Chen, H. Cai,
and B.C. Cai, Fitoterapia, 81 (2010)
1045-1052.