Õèìèÿ è õèìè÷åñêèå òåõíîëîãèè/5.Ôóíäàìåíòàëüíûå ïðîáëåìû
ñîçäàíèÿ íîâûõ ìàòåðèàëîâ è òåõíîëîãèé.
Viazovik V.N., Yarovoi R.V., Stolyarenko G.S.
Burning of
coal and wood using electrocatalysis
The
development of civilization continues to set new tasks for science, even in
traditional areas that have been established. And one of the major global
challenges of modern civilization is the fact that every year the need for
energy increases, and the world's reserves of fuel for thermal power decrease,
and adequate alternative power system, which would satisfy mankind needs has
found.
This
direction is important, due to the relatively low reactivity of natural
hydrocarbons, and therefore the combustion process in modern combustion
chambers are not fully completed and released into the atmosphere large amount
of environmentally harmful products of incomplete combustion.
One
of the most promising solutions to this problem may be the transition to the
new principles of burning hydrocarbons. One of these promising methods is electrocatalysis combustion of fuels based on the
initiation of the combustion process on the catalyst in an electric discharge.
Theoretical framework.
With heating (below the ignition temperature of the
solid fuel) there is thermal decomposition of the organic mass of fuel,
emitting volatile substances, which include a significant amount of
combustibles, such as methane, hydrogen, carbon dioxide (II) and so on. They
pretty much determine conditions of inflammation and combustion.
Using
the method of electrocatalysis intensification of combustion of solid fuel can
increase the yield of volatile compounds, their composition contains volatiles
with heating value is significantly higher than the heat of combustion of
compounds that are formed during normal thermolysis. Also electrocatalysis
leads to the formation of volatile compounds at much lower temperatures,
allowing using excess heat that is formed on the target needs.
The
next stage of the combustion of solid fuels is the burning of the coke, which
is composed of pure carbon, which is connected to a polycrystalline structure/
Using electrocatalysis besides the usual process of combustion of carbon, the
process of "shaking" of the crystal structure of coke, breaks the
bonds between the carbon atoms, which in turn improves the process of mass
transfer of oxygen and combustion products. This increases the fuel burn and
heat the target needs.
And
another feature of electrocatalysis is that in the first stages of the process
of combustion of the fuel, a large amount of moisture is released. The water
molecule, while it is in the area of electric discharge and the strong
electromagnetic field (which is always present in any form of electrical
discharge) dissociates with reaction:
H2O + e →HO![]() + H +e. (1)
+ H +e. (1)
Molecular
oxygen in ordinary combustion is destroyed by the heat of reaction:
Î2→Î+Î (2)
In
the area of the discharge oxygen molecule is destroyed rapidly and form atomic
oxygen, which is necessary for the oxidation of volatile compounds and carbon
coke, also for this process there is no need for high temperatures and can
occur under normal conditions. The output of the oxygen atoms from the reaction
(2) using electrocatalysis is significantly higher than using normal
combustion. Therefore, it becomes believable flow of following reactions:
HO![]() + Î→ÍÎ
+ Î→ÍÎ![]() (3)
(3)
Í + Î→ HO![]() (4)
(4)
These
oxygen radicals are always present in combustion and significantly affect the
burning process. Particularly significant impact on the combustion process has
radical ÍÎ![]() in combustion of volatile compounds, and is the cause
of the third limit switch.
in combustion of volatile compounds, and is the cause
of the third limit switch.
The results of the experiments. Experiments to optimize the combustion of solid
fuels (coal and wood) were conducted at the facility, which is compounded: from
the burning chamber with discharge device, thermostat, power supply and
Table 1 - The results of intensification coal combustion
|
¹ |
Number of electrodes |
Voltage, kV |
The average value of heat increasing, % |
Changes in the degree of burnout, % |
|
1 |
Design ¹1 |
8 |
7,64 |
Decreased by 25% |
|
2 |
Design ¹2 |
8 |
8,56 |
Decreased by 16% |
|
3 |
Design ¹2 |
4 |
20,13 |
Increased by 32 % |
|
4 |
Design ¹2 |
2,8 |
7,18 |
Increased by 24,9 % |
|
5 |
Design ¹3 |
2,8 |
11,05 |
Increased by 30 % |
compressor discharge gap, a constant
volume of water, which is heated. Experiments were performed with both electrocatalysis
processing, and without it. During experiments every two minutes the temperature
of heated water was measured.
For
coal there were used several designs of electrocatalysis devices. They differed
in designs and operating modes. The results are presented in Table 1.
Intensification
of combustion of solid fuel is affected significantly by the voltage and design
of electrocatalysis device. Voltage in the electrocatalysis device represents
between 2.8 to 8 kV. Carrying out the process with such conditions the fuel
savings up to 7.64% was achieved.
Significant
impact on process of intensification of burning solid fuel has discharge
voltage. It was found that the optimal voltage for enhanced combustion is 4 kV
and achieved average fuel economy of 20%. At higher voltages (8 kV), average
fuel economy value decreases to 8.56%. Also high effect observed at much lower
voltages. So at a voltage 2.8 kV average fuel economy reaches 7.18%.
Electricity
costs for all of the studies were 25-30 W·Hour on burning 1 kg of coal.
It
was also studied the change in the burn-up of fuel. As can be seen from Table 1
at different ways of handling different values achieved increasing the burn-up
of fuel. With Design 1 construction voltage of 8 kV and structure 2 and the
same voltages the burn-up reduced and represented 25 and 16%. The greatest
increase in the burn-up of fuel is in the processing of a single electrode and
a voltage of 4 kV and represented 30%. For other modes, the rate of fuel burn
increase was lower.
Similar
studies were made for brown coal. Was investigated combustion heat effect of
the fuel with and without the use of electrocatalysis device, and time of
release volatile compounds.
In
Fig. 1 shows the change in water temperature by burning brown coal with and
without treatment. As can be seen from Figure 5 there is a significant increase
in the intensification of the heat combustion of brown coal and it reaches
21.66%. The degree of burnout of coal after electrocatalysis device is
increased from 75% to 80%. The yield of volatile time increases from 55 to 70
s, which is about 27%.
Using
the results of research on coal combustion using electrocatalysis we studied
burning of wood and pellets. In Fig. 2-3 here is some
results of burning wood using electrocatalysis.
So by using electrocatalysis with
wood, namely, pine and birch, the largest difference in temperature between the
control experiment and experiment with treatment, is achieved at a voltage of
3.5 - 5 kV to 500 g of wood can reach 2,5-3 º C (heating value is
increased to 71-75%). At lower voltages, the difference is much smaller, and
the temperature is 1,5 º C (about 27%). For voltages greater than 5 kV, a
gradual reduction of the difference and it does not exceed 1-1,5 º C
(15-20%).
For pine at a voltage 3.1 -3.5 kV can reach 1-1.5 (14-21%). For voltages
greater than 3.5 kV, a gradual decrease and it is not above 0,5 º C (7%).
The greatest power effect is achieved at a voltage of 3 to 5 kV. When the
voltage increases the energy effect decreases.
Analyzing the percentage of energy used for electrocatalysis with the
amount of energy that can be produced from the burning of wood, we can see that
at voltages 3-3.5 kV percent is lower and no more than 2-2.3%. At higher
voltages this percentage is growing at 8.7 kV voltages and does not exceed 4%.
But with all the stresses that despite the reduction of the energy effect, this
percentage does not exceed the amount of excess energy, which is allocated with
the use of electrocatalysis.
When burning
pellets, there is such results (see Fig. 4). Maximum temperature
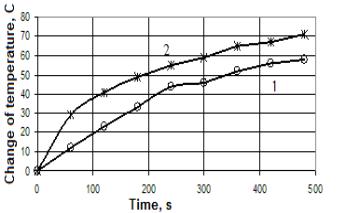
Fig.
1 - Dependence of water temperature changes from time during combustion
brown coal.
1
- without treatment, 2 - with treatment.
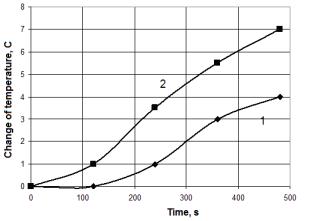
Fig. 2. Dependence of the change temperature from time by burning birch wood.
1 - no treatment,
2 – with treatment.
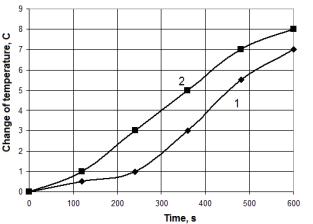
Fig. 3. Dependence of the temperature change from time by burning pine wood.
1 - no treatment, 2 – with
treatment.
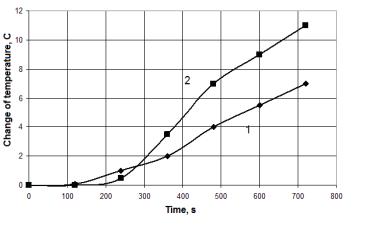
Fig. 4. Dependence of the
temperature change from time by burning pellets.
1 - no treatment, 2 – with treatment.
difference between
the control experiment and experiment with treatment achieved at a voltage of 5
kV and exceeds up to 4 º C (500 g pellets). At lower voltages (3-4 kV),
this difference is less. So at a voltage of 3 kV is less than 0,5 º C,
which is no more than the experimental error. At a voltage of 4 kV the
difference is already 2 º C. For voltages greater than 5 kV power effect
is reduced to 2 º C and remained stable.
The highest energy effect, as well as for the wood, is achieved at a
voltage of 3 to 5 kV. At these voltages, the percentage of energy used on the
process of electrocatalysis doesn’t exceed 1.5%. When the voltage increases the
energy effect decreases.
This delivers a significant reduction in emissions. With coal combustion
NOx emissions reduction is achieved by 80%, CO by 52%. With burning
of wood – NOx reduces by 49%, CO by 33%.
Conclusion. The conclusion is that using of electrocatalysis with burning solid
fuels, namely coal, firewood and pellets leads to a significant increase in the
amount of heat: the coal to 10-12%, wood and pellets to 71-75%. This delivers a
significant reduction in environmental impact. So with coal combustion NOx
emissions reduction is achieved by 80%, CO by 52%. Using wood -reduces NOx
by 49%, CO by 33%. The degree of burnout of coal increases by 17.5%. The
consumption of energy to undertake the process does not exceed 5% of the excess
heat.