Chemistry and Chemical Technologies / 4. Chemical and
Pharmaceutical Industry
Dr. Valery V. Belakhov1, Dr. Sc. Yury D.
Shenin2, Dr. Sc.,
Prof. Aleksandr V. Garabadzhiu3a, Dr. Sc.,
Prof. Boris I. Ionin3b
1Schulich Faculty of Chemistry, Technion Israel Institute
of Technology, Haifa, Israel; 2All-Russian
Research Institute of Plant Protection, Saint-Petersburg, Russia; 3aDepartment
of Technology of Microbiological Synthesis and
3bDepartment of Organic Chemistry, Saint-Petersburg State
Technological Institute
(Technical
University), Saint-Petersburg, Russia
chvalery@techunix.technion.ac.il
CHEMICAL MODIFICATION OF
POLYENE MACROLIDE ANTIBIOTICS AS A TOOL FOR THE PREPARATION OF NOVEL LOW-TOXIC
DERIVATIVES WITH AN EXTENSIVE SPECTRUM OF BIOLOGICAL ACTIVITY
1. Introduction
Polyene macrolide antibiotics (PMA)
constitute an abundant group of natural compounds that embarrasses over 200
preparations revealing activity toward yeast, yeast-like, and filamentous fungi
of both saprophytic and pathogenic species [1-4]. Perspectives of medical
application of PMA were enhanced after revealing their antiviral activity
[5-8], activity toward parasitic Protozoa [9-11], and potential use as
antitumor agents [12-14]. They are particularly useful for treatment of fungal
diseases complicating AIDS [15-18]. On the other hand, PMA used in medical
practice do not satisfy completely requirements of clinical physicians due to
restricted efficiency, in part, inapplicability for treatment of certain forms
of deep mycoses [19-21]. Medical application of these antifungal preparations
is also hindered by their high toxicity [22-25], instability on storage
[26-30], poor solubility in water [1-4], and reduced sensitivity of these
antibiotics to pathogenic fungal microorganisms [31-34]. Therefore, search for
new derivatives of PMA with improved chemotherapeutic properties is being
continued.
2.
Results and Discussion
One of the promising methods of chemical
modification of drugs and, in particular, PMA is hydrophosphorylation [3537].
Our studies on the synthesis of semi-synthetic PMA derivatives have included
the reactions of PMA with aromatic aldehydes and hypophosphorous acid, and an
investigation of medical and biological properties of the obtained derivatives.
Thus, it was interesting to prepare the hydrophosphoryl derivatives of PMA to
examine their biomedical properties and to conduct a comparative analysis of
their biological effectiveness with initial PMA.
We showed that PMA levorin, nystatin,
amphotericin B, mycoheptin, pimaricin and lucensomycin react with aromatic
aldehydes and hypophosphorous acid with the formation of hydrophosphoryl
derivatives of these antifungal antibiotics [38-43]. The studied reaction can
be considered as a specific version of the Kabachnik-Fields reaction [44, 45]
whose synthetic performance has been considered in detail in [46, 47]. In the
first step of the process, the primary amino group of the pimaricin
carbohydrate fragment is added to the carbonyl group of the aromatic aldehyde to
form an azomethine intermediate. In the second step, hypophosphorous acid
reacts at the C=N bond of the azomethine fragment to afford hydrophosphoryl
derivatives of PMA. The general scheme of this reaction was performed for
tetraene macrolide antibiotic pimaricin.
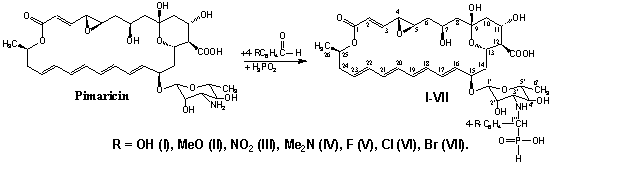
As phosphorylating compound we used
hypophosphorous acid, which is soft hydrophosphoryl reagent [48]. We used the
following aldehydes: 4-hydroxybenzaldehyde, 4-methoxybenzaldehyde,
4-nitrobenzaldehyde, 4-(dimethylamino)benzaldehyde, 4-fluorobenzaldehyde,
4-chlorobenzaldehyde, and 4-bromobenzaldehyde. The
hydrophosphoryl derivatives of PMA are
solid crystalline compounds having no sharp melting point and decomposing on
heating. These compounds are readily soluble in dimethylsulfoxide and
dimethylformamide, moderately soluble in methanol, ethanol, and water,
insoluble in chloroform, higher alcohols, ethers, dioxane, benzene and hexane.
The biological tests
demonstrated that acute toxicity (LD50) of hydrophosphoryl derivatives
of PMA was 26 times lower than that of original antibiotics
[38-43].
Biological investigations showed that hydrophosphoryl
derivatives of PMA possess a high antifungal activity toward 21 pathogens of different mycotic infections and, especially,
to yeast-like fungi of the genus Candida (C.
albicans, C. utilis, C. tropicalis, C. krusei, C. parapsilosis,
C. guillermondii).
It is known that
chemical modification of biologically active compounds in some cases leads to a
change in a range of biological activity of obtained derivatives and to
reducing their toxicity [35, 36, 49-53]. Previously, various authors have found
antiviral and antitumor activity which is nonspecific for polyene macrolide
antibiotics [1214]. On the base of a detailed study of molecular and
biological mechanisms of lipido-membranotropic effect of PMA was formulated the
hypothesis that these antifungal preparations possessed an antiviral activity
[5-8]. According to this hypothesis, PMA cause a reorientation of the lipid matrix
surface-membrane or virus-specific receptors of the cellular cytoplasmatic
membrane, thereby, inactivating enveloped (lipid-containing) viruses or
preventing the penetration of virus into sensitive cells. This hypothesis was
later confirmed experimentally at the molecular and sub-cellular levels
[54-58]. Hence, the mechanism of an antiviral action of PMA includes two points
of application: 1) an interaction between PMA and lipids of cellular
cytoplasmic membranes, which leads to a change in membrane permeability and to
an antiviral activity; and 2) an interaction between PMA and lipids of the
virion envelope, which appears as a viricidal effect of these antifungal
agents. It was shown that the mechanism of antiviral action of PMA differs
substantially from the virus-inhibitory effect of antibacterial non-polyene
antibiotics [59]. Therefore, it was interesting to study the antiviral activity
of hydrophosphoryl derivatives of PMA.
The study of the toxicity of
hydrophosphoryl derivatives of PMA
for 1011-day-old developing chick
embryos showed that these compounds are less toxic than the original PMA.
Antiviral activity of synthesized derivatives of PMA against model viruses was
studied in experiments in
ovo with doses 1/5 LD50. Biological studies showed
that several hydrophosphoryl derivatives of PMA had high activity in relation
to vaccinia virus and to types A and B influenza viruses [38-43].
Data obtained in the study of
hydrophosphoryl derivatives of PMA by a model of RNA-containing Rous sarcoma
retrovirus are of particular interest, since this model is suggested as an
adequate retroviral model for screening and studying drugs against AIDS [8, 60,
6]. It was found that a series of studied derivatives of PMA demonstrated high
antiviral activity against RSV both in prophylactic and therapeutic schemes of
the injection [38-43].
3.
Experimental part
3.1. Experimental physical and
chemical part
The structure of these derivatives was
confirmed using 1H, 13C, and 31P NMR, as well
as IR and UV spectroscopy. The 1H and 13C NMR spectra
were obtained on a Bruker Avance instrument (Germany) at 500 MHz (for protons)
for 10-15% solutions in DMSO-d6 or
MeOH-d4, internal reference TMS. The 31P NMR
spectra were registered on a Bruker AC-200 instrument (Germany) with operating
frequency 80 MHz (200 MHz for protons), external reference 85% H3PO4.
The IR spectra were registered on a Bruker Vector 22 spectrophotometer
(Germany) from KBr pellets. The UV spectra were recorded on an Ultrospec 2100
pro instrument (Biochrom, UK). Individuality of hydrophosphoryl derivatives of
PMA was verified by TLC on Silica Gel 60 F254 plates (0.25
mm, Merck, Germany) in corresponding systems; spots were developed by UV
illumination. Column chromatography was performed on Silica Gel 60 (63-200 mm, Merck, Germany).
3.2. Experimental medical and
biological part
Acute toxicity (LD50) of
hydrophosphoryl derivatives of PMA
was studied on white mongrel male mice
weighing 1820 g. The compounds were diluted with 0.5% aqueous solution of
carboxymethylcellulose and tested in the form of suspensions by intraperitoneal
injection. The LD50 values were
calculated from the test results using the Kerber method.
Antifungal activity of hydrophosphoryl derivatives of PMA against 21 pathogens of different mycotic infections was determined using serial dilutions in
liquid nutrient medium. The method was based on successive two-fold dilutions
of the tested compounds. The minimal fungistatic concentration (MFC) was
established by a visual assessment of the growth of test cultures in
experimental and control tubes using three repetitions.
The toxicity of 1011
day developing chick embryos was determined by introducing the compounds into
the allantoic cavity or horionallantoic membrane (HAM) before examining the
antiviral action of hydrophosphoryl derivatives of PMA. In each dilution we
used six embryos which were incubated at 37°C until hatching with ovoscoping
every two days. Antiviral activity of these compounds in experiments was examined
by in ovo technique against DNA-containing variolovaccine virus using
a L-IVP 01.72 strain. Working titers of variolovaccine virus were 106108
ODE ml1. Virus-containing material was prepared in a McIlavaines
solution. The test compounds were administered at the HAM of 1011-day-old
chick embryos according to prophylactic (for 1 h prior to the introduction of
the virus) and therapeutic schemes (1 h after infection). Incubation of embryos
was carried out for 4872 h at 37°C. Counting of the pocks was performed on HAM
of embryo. Antiviral activity of hydrophosphoryl derivatives of PMA was examined against RNA containing viruses, oncogenic Rous
sarcoma virus (RSV) [(strain RSV (RSV-1)], and infectious virus of type A ([a
strain A2 Odessa 2882/82 (H3N2)] and type B ([a strain B/USSR (69)]
in developing chick embryos. After determining the toxicity of compounds
1011-day-old developing chick embryos were administered in the test with the
RSV on the HAM according to prophylactic and therapeutic schemes. Number of
foci of neoplastic transformation in the HAM of embryos was counted on 8th day
after incubation at 37°C. Determination of antiinfluenzal action were carried
out by the introduction of derivatives of hydrophosphoryl derivatives of PMA in the horionallantoic cavity of 1011-day-old developing
chick embryos by prophylactic scheme, i.e., 10100 EID50 of virus
for 11.5 h before incubation. The results were taken into account after 48 h
of incubation of embryos at 37°C in the case of influenza virus of type A and
after 72 h at 34°C in the case of influenza virus of type B. The presence of
virus in embryos were determined in the reaction of hemagglutination with
chicken red blood cells in the allantoic fluid of experimental and control
embryos. An isotonic sodium chloride solution was injected into the control
embryos instead of substances under study. Antiviral activity of the tested
compounds in experiments in
ovo was assessed by a protection index
(PI), which was calculated by the following formula:
amount of virus-infected amount of virus-infected
in control embryos
(%) ‾ in experiment
embryos (%)
PI =
× 100.
amount of
virus-infected in control embryos (%)
4.
Conclusions
1. The hydrophosphoryl
derivatives of PMA were successfully prepared under the Kabachnik-Fields
reaction conditions.
2. The studied reactions can be
considered as a general method for chemical modification of polyene macrolide
antibiotics via hydrophosphorylation.
3. This method opens promising
direction for the preparation of novel low-toxic derivatives of PMA with
improved medical and biological properties and extensive range of biological
activity.
5.
Acknowledgments
This work was supported by the Ministry of
Education and Science of Russian Federation under the framework of Federal
Program "Scientific and Educational Specialists of the Innovational
Russia" during 2009-2013 on the topic: "Development of new semi-synthetic
derivatives and modification of known
polyene macrolide antibiotics for the preparation of highly effective
antifungal drugs" (grant No. 2012-1.5-12-000-1013-005).
Literature
1. Omura
S., Tanaka H., Macrolide Antibiotics: Chemistry, Biology and Practice, Omura,
S., Ed., New York: Academic, 1984, p. 351-404.
2. Bader
G., Deicke P., Gedek B., Gemeihardt H., Muller J., Sholer H., Spitzbart H.,
Wiesner B., Endomykosen: Schleimhaut - Organ- and Systemmykosen Herausgegeben von Horst Gemeinhardt, Berlin:
Gustvtischer, 1989, 510 S.
3. Antifungal
Drug Therapy (A Complete Guide for the Practitioner), Jacobs P.H., Nall L.,
Eds., New York: Dekker, 1990, 368 P.
4. Recent
Progress in Antifungal Chemotherapy, Yamaguchi H., Kobayashi G.S.,
Takahashi H., Eds., New York: Dekker, 1992, 765 P.
5. Shneider
M.A., in: Uspekhi v oblasti izucheniya I proizvodstva antibiotikov (Advances
in Studying and Manufacturing antibiotics), Moscow, pp. 56-69, 1979.
6. Shneider
M.A., Shtilbans E.B., Rachkovskaya L.A., Poltorak V.A., Antibiotiki,
vol. 28, No. 5, pp. 352-357, 1983.
7. Shneider
M.A., Molek. Genet., Mikrobiol. Virusol., No. 5, pp. 41-46, 1984.
8. Shneider
M.A, Chizhov N.P., Vopr. Virusol., vol. 31, No. 1, pp. 18-31, 1986.
9.
Ouellette M., Drummelsmith J., Papadopoulou B., Drug Resist. Updates,
vol. 7, No. 4-5, pp. 257-266, 2004.
10.
Goldsmith D.R., Perry C.M., Drugs, vol. 64, No. 17, pp. 1905-1911, 2004.
11.
Golenser J., Domb A., Mini Review Med. Chem., vol. 6, No. 2, pp.
153-162, 2006.
12.
Vertut-Croquin A., Brajtburg J., Medoff, G., Cancer Res., vol. 46, No. 12, pp. 6054-6058, 1986.
13. Coune
A., Eur. J. Cancer Clin. Oncol., vol. 24, No. 2, pp. 117-121, 1988.
14. Feigin
A.M., Med. Hypotheses., vol. 52, No. 5, pp. 383-388, 1999.
15.
Kirkpatrick C.H., AIDS: Anti-HIV Agents, Therapies and Vaccines (Annals of
New York Academy of Sciences), Georgiev V.S., McGowan J.J., Eds., New
York, vol. 616, pp. 461-468, 1990.
16. Vyadro
M.M., Antibiot. Khimioter., vol. 36, No. 11, pp. 45-48, 1991.
17.
Ablordeppey S.Y., Fan, P., Ablordeppey J.H., Mardenborough L., Curr. Med.
Chem., vol. 6, No. 12, pp. 1151-1195, 1999.
18. Hood
S., Denning D.W., J. Antimicrob. Chemother., vol. 37, Suppl. B, pp.
71-85, 1996.
19. Gallis
H.A., Drew, R.H., Rev. Infect. Dis., vol. 12, No. 2, pp. 308-329, 1990.
20.
Georgopapadakou N.H., Walsh, T.J., Antimicrob. Agents Chemother., vol.
40, No. 2, pp. 279-291, 1996.
21. Grillot
R., Lebeau B., Antimicrobial Agents, Bryskier A., (Ed.), Washington:
Publ. Am. Soc. Microbiol., pp. 1260-1287, 2005.
22. Fanos,
V. and Cataldi, L., J. Chemother., vol. 12, No. 6, pp. 463-470, 2000.
23. Deray
G., J. Antimicrob. Chemother., vol. 49, Suppl. S1, p. 37-41, 2002.
24.
Bernardo J.F., Sabra R., Branch R.A., Clinical Nephrotoxins, De Broe
M.E., (Ed.), Dordrecht: Kluwer Academic, pp. 199-222, 2003.
25. Wingard
J.R., Leather H., Biol. Blood Marrow Transplant., vol. 10, pp. 73-90,
2004.
26. Thomas
A.H., Analyst, vol. 101, No. 1202, pp. 321-340, 1976.
27. Shenin
Yu.D., Belakhov V.V., Gagarina A.B., Kasaikina O.T., and Vainshtein V.A., Polienovye
makrolidnye antibiotiki: inaktivatsiya i sposoby ikh stabilizatsii (Polyene
Macrolide Antibiotics: Inactivation and Methods of Stabilization), Review
Information, serried "Remedies, Economics, Technology, and Perspectives of
Producing", Moscow: VNIISENTI, 1990, No. 12, 36 P.
28. Shenin
Yu.D., Belakhov V.V., Koptelova T.N., Levina A.A., Shchinova N.E., Antibiot.
Khimiot., vol. 36, No. 8, pp. 20-24, 1991.
29.
Lamy-Freund M.T., Ferreira V.F., Schreier S., J. Antibiot., vol. 38, No.
6, pp. 753-757, 1985.
30.
Belakhov V.V., Shenin Yu.D., Nifantev E.E., Kukhareva T.S., Soldatova I.A., Antibiot.
Khimioter., vol. 42, No. 12, pp. 6-9, 1997.
31.
Belousova I.I., Antibiotiki, vol. 21, No. 7, pp. 661-667, 1976.
32. Kaufman
C.H., Carver, P.L., Drugs, vol. 53, No. 4, pp. 539-549, 1997.
33. Bossche
H.V., Dromer F., Improvisi, I., Lozano-Chiu M., Rex J.H., Sanglard D., Med.
Mycol., vol. 36, No. 1, pp. 119-128, 1998.
34. Stevens
D.A., Holmberg K., Curr. Opin. Anti-Infect. Invest. Drugs, vol. 1, No.
3, pp. 306-317, 1999.
35. Yudelevich
V.I., Komarov E.V., Ionin B.I., Khim.-Farm. Zh., vol. 19, No. 6, pp.
668685, 1985.
36.
Yudelevich V.I., Komarov E.V., Ionin B.I., In Fosfororganicheskie i
kremnii-organicheskie soedineniya (Organo-phosphorus and Organo-silicon
Compounds), Leningrad, pp. 104133, 1985.
37.
Corbridge D.E.C., Phosphorus, an Outline of Its Chemistry, Biochemistry and
Technology, Amsterdam: Verlag Elsevier Scientific Publishing
Company, 2000, P. 925-1099.
38.
Belakhov V.V., Shenin Yu.D., Ionin B.I., et al., Antibiotiki i Khimioter.,
vol. 35, No. 8, pp. 31- 35, 1990.
39.
Belakhov V.V., Ionin B. I., Shenin Yu. D., et al., Pharm. Chem. J., vol.
25, No.11, pp. 809 813, 1991.
40.
Belakhov V.V., Shenin Yu.D., Araviiskii R.A., Shtilbans E.B., Antibiotiki i
Khimioter., vol. 41, No. 7- 8, pp. 48, 1998.
41.
Belakhov V.V., Shenin Yu. D., Pharm. Chem. J., vol. 41, No.6, pp. 314
318, 2007.
42.
Belakhov V.V., Shenin Yu.D., Ionin B.I., Russ. J. General Chem., vol.
78, No. 2, pp. 305-312, 2008.
43.
Belakhov V.V., Kolodyaznaya
V.A., Ionin B.I. Russ. J.
Appl. Chem., vol. 85, No. 9, pp. 1454−1465, 2012.
44.
Kabachnik M.I., Medved, T.Ya., Dokl. Akad. Nauk SSSR, vol. 83, No. 3,
pp. 689691, 1952.
45. Fields
E.K., J. Am. Chem. Soc., vol. 74, No. 6, pp. 15281531, 1952.
46.
Nifantev E.E., Khimiya gidrofosforilnykh soedinenii (Chemistry of Hydrophosphoryl
Compounds), Moscow: Nauka, pp. 5559, 1983.
47.
Cherkasov R.A., Galkin V.I., Usp. Khim., vol. 67, No. 10, pp. 940968,
1998.
48.
Yudelevich V.I., Sokolov L.B., Ionin B.I., Usp. Khim., vol. 49, No. 1,
pp. 92117, 1980.
49.
Yudelevich V.I., Ionin B.I., Fosfororganicheskie lekarstvennye preparaty (Organo-Phosphorus
Drugs), St. Petersburg: Teza, 1995, P. 58-65.
50. Shenin
Yu.D., Belakhov V.V., Khimiya polienovykh makrolidnykh antibiotikov (Chemistry
of Polyene Macrolide Antibiotics), Review Information, series
"Remedies, Economics, Technology, and Perspectives of
Production", Moscow: VNIISENTI, 1989, No. 6, 44 P.
51.
Belakhov V.V., Yudelevich V.I., Ionin B.I., et al., Russ.
J. General Chem., vol. 58, No. 6,
pp. 10591070, 1988.
52. Shenin
Yu.D., Belakhov V.V., Araviiskii R.A., Pharm.
Chem. J., vol. 27, No. 2, pp. 8492, 1993.
53. Shenin
Yu.D., Belakhov V.V., Antibiot. Khimioterap., vol. 42, No. 4, pp. 3446,
1997.
54. Oksman
A.Ya., Shneider M.A., Asinovskaya N.K. et al., Eksperimental'naya Onkologiya,
vol. 3, No. 4, pp. 37-41, 1981.
55.
Shneider M.A., Stilbans E.B., Gavrilov G.A., Antibiotiki, vol. 27, No.
9, pp. 693-697, 1982.
56.
Shneider M.A., Oksman A.Ya., Byulleten Eksperimental'noi Biologii i
Meditsiny, vol. 95, No. 3, pp. 63-66, 1983.
57. Shneider
M.A., Molekulyarnaya Genetika,
Mikrobiologiya i Virusologiya, No. 5, pp. 41-46, 1984.
58.
Shneider M.A., Stilbans E.B., Rachkovskaya L.A. et al., Antibiotiki,
vol. 29, No. 5, pp. 344-349, 1982.
59. Lancini
G., Parenti F. Antibiotics. An Integrated View. New York:
Springer-Verlag, 1982, 253 P.
60. Shneider M.A., Rudenko N.K., Kavsan V.M.,
Bibilashvili R.Sh., Molek. Biol., vol. 21, No. 3, pp. 837846, 1987.
61. Kavsan V.M., Rudenko N.K., Shneider M.A. et al., Doklady
Akademii Nauk SSSR, vol. 296, No. 6, pp. 1492-1497, 1987.
SUMMARY
V.V. Belakhov1, Y.D. Shenin2,
A.V.Garabadzhiu3a, B. I. Ionin3b
1Schulich Faculty of Chemistry, Technion Israel
Institute of Technology, Haifa, Israel; 2All-Russian Research
Institute of Plant Protection, Saint-Petersburg, Russia; 3aDepartment
of Technology of Microbiological Synthesis and 3bDepartment of
Organic Chemistry, Saint-Petersburg State Technological Institute (Technical
University), Saint-Petersburg, Russia
CHEMICAL MODIFICATION OF
POLYENE MACROLIDE ANTIBIOTICS AS A TOOL FOR THE PREPARATION OF NOVEL LOW-TOXIC
DERIVATIVES WITH AN EXTENSIVE SPECTRUM OF BIOLOGICAL ACTIVITY
Reactions
of the polyene macrolide antibiotics with aromatic aldehydes and
hypophosphorous acid yielded their hydrophosphoryl derivatives. The
physicochemical and biological properties of these semi-synthetic derivatives
were studied. Biological investigations showed that hydrophosphoryl derivatives
of polyene macrolide antibiotics were less toxic than the initial antibiotic
and had antifungal and antiviral activity.
Keywords: polyene macrolide antibiotics, hydrophosphoryl derivatives, antifungal and antiviral activity, toxicity.