| |
|
|
|
|
| 8 |
|
|
|
|
| 6 |
|
|
|
|
| 4 |
|
|
|
|
| 2 |
|
|
|
|
| 0 |
|
|
|
|
| 300 |
320 |
340 |
360 |
380 |
| |
|
|
|
T, K |
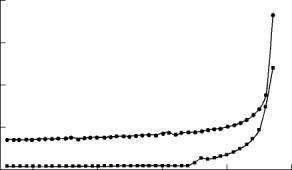
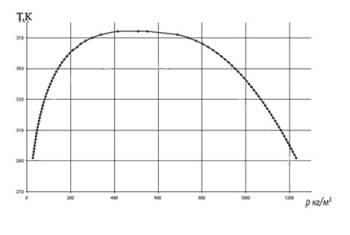
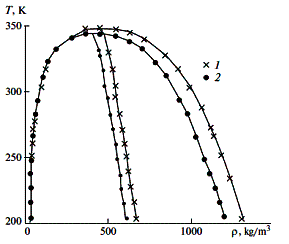
| |
|
|
|
|
| 8 |
|
|
|
|
| 6 |
|
|
|
|
| 4 |
|
|
|
|
| 2 |
|
|
|
|
| 0 |
|
|
|
|
| 300 |
320 |
340 |
360 |
380 |
| |
|
|
|
T, K |



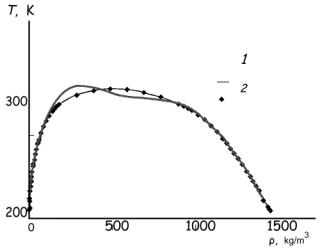
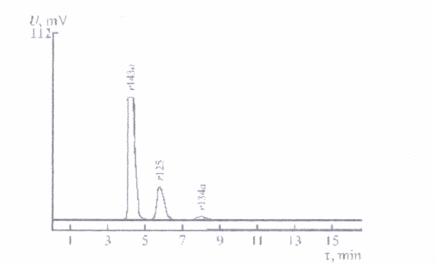
| Cooling agent |
–Tb, °Ñ
[1] |
Òc
, Ê |
ρc , kg/m3 |
| |
|
|
|
| R 134à |
26.2 |
374.18 [5] |
508.00 [5] |
| R 401À |
33.1 |
381.25 |
510.60 |
| R 402À |
49.2 |
349.00* |
500.00* |
| R 404À |
46.5 |
345.20* |
485.00* |
| R 407À |
45.5 |
351.00* |
500.00* |
| |
|
|
|
|
Refrigerants |
Formula |
Tboili,°C |
Tcritical, K |
Pcritical, MPa |
ρcritical, kg/m3 |
|
R218 R227ea R227ca R236ea R236fa R245ca R290 R1270 Ethyl
methyl ether Trimethylamine |
CF3–CF2–CF3 CF3–CHF–CHF3 CF3–CF2–CHF3 CF3–CHF–CHF2 CF3–CH2–CF3 CF3–CF2–CF2 C3H8 C3H6 C3H8O C3H9N |
–36.1 –17.3 –16.0 6.5 –0.7 25.0 –41.6 –47.73 7.45 3.0 |
345.05 375.04 – 412.4 – – 369.85 365.05 437.8 432.79 |
2.677 2.930 – 3.412 – – 4.248 4.640 4.430 4.087 |
628.0 589.99 – – – – 227 ± 2 230.0 272.0 232.7 |
|
|
|
|
|
|
||||||||||||||||
| Table. 3.
Thermodynamic properties of
HFC 227ea Freon |
|
|
|
|
|||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|||||||||||
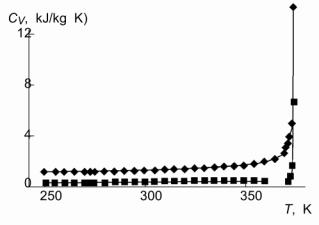
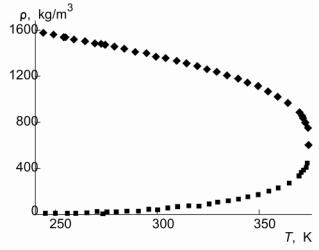
| T, K |
ρ' . 10 |
–3 |
kg/m3 |
|
ρ'' . 10 |
–3 |
,kg/m3 |
|
C'V
, kJ/(kg K) |
C"V , kJ/(kg K) |
|
||||||||||
| |
|
|
|
|
|||||||||||||||||
| |
|
|
|
|
|
|
|||||||||||||||
| 245.15 |
1.5842 |
|
0.0051675 |
|
1.210 |
0.2669 |
|
||||||||||||||
| 250.15 |
1.5677 |
|
0.0064448 |
|
1.219 |
0.2893 |
|
||||||||||||||
| 255.15 |
1.5508 |
|
0.0079646 |
|
1.228 |
0.3093 |
|
||||||||||||||
| 256.728 |
1.5454 |
|
0.0084998 |
|
1.236 |
0.3229 |
|
||||||||||||||
| 260.15 |
1.5336 |
|
0.00975954 |
|
1.241 |
0.3308 |
|
||||||||||||||
| 265.15 |
1.5158 |
|
0.0118630 |
|
1.249 |
0.3431 |
|
||||||||||||||
| 270.15 |
1.4977 |
|
0.0143120 |
|
1.261 |
0.3398 |
|
||||||||||||||
| 273.15 |
1.4866 |
|
0.0159650 |
|
1.271 |
0.3695 |
|
||||||||||||||
| 275.15 |
1.4791 |
|
0.0171480 |
|
1.278 |
0.3743 |
|
||||||||||||||
| 280.15 |
1.4601 |
|
0.0204110 |
|
1.288 |
0.3843 |
|
||||||||||||||
| 285.15 |
1.4405 |
|
0.0241510 |
|
1.303 |
0.3969 |
|
||||||||||||||
| 290.15 |
1.4204 |
|
0.0284210 |
|
1.318 |
0.4091 |
|
||||||||||||||
| 295.15 |
1.3997 |
|
0.0332800 |
|
1.336 |
0.4203 |
|
||||||||||||||
| 300.15 |
1.3785 |
|
0.0387970 |
|
1.355 |
0.4317 |
|
||||||||||||||
| 305.15 |
1.3566 |
|
0.0450500 |
|
1.376 |
0.4419 |
|
||||||||||||||
| 310.15 |
1.3349 |
|
0.0521060 |
|
1.399 |
0.4510 |
|
||||||||||||||
| 315.15 |
1.3102 |
|
0.0601560 |
|
1.426 |
0.4596 |
|
||||||||||||||
| 320.15 |
1.2857 |
|
0.0692520 |
|
1.455 |
0.4670 |
|
||||||||||||||
| 325.15 |
1.2600 |
|
0.0795870 |
|
1.488 |
0.4737 |
|
||||||||||||||
| 330.15 |
1.2329 |
|
0.0913680 |
|
1.528 |
0.4784 |
|
||||||||||||||
| 335.15 |
1.2043 |
|
0.1048600 |
|
1.572 |
0.4810 |
|
||||||||||||||
| 340.15 |
1.1737 |
|
0.1204400 |
|
1.626 |
0.4815 |
|
||||||||||||||
| 345.15 |
1.1405 |
|
0.1385900 |
|
1.692 |
0.4770 |
|
||||||||||||||
| 350.15 |
1.1041 |
|
0.1600600 |
|
1.774 |
0.4663 |
|
||||||||||||||
| 355.15 |
1.0632 |
|
0.1860000 |
|
1.883 |
0.4458 |
|
||||||||||||||
| 360.15 |
1.0155 |
|
0.2184000 |
|
2.035 |
0.4049 |
|
||||||||||||||
| 365.15 |
0.9566 |
|
0.2613000 |
|
2.269 |
– |
|
||||||||||||||
| 370.15 |
0.8739 |
|
0.3258600 |
|
2.714 |
– |
|
||||||||||||||
| 371.15 |
0.8512 |
|
0.3258600 |
|
3.231 |
– |
|
||||||||||||||
| 372.15 |
0.8243 |
|
0.3663200 |
|
3.555 |
0.4276 |
|
||||||||||||||
| 373.15 |
0.7905 |
|
0.3945300 |
|
4.058 |
0.8279 |
|
||||||||||||||
| 374.15 |
0.7417 |
|
0.4360300 |
|
5.039 |
1.7346 |
|
||||||||||||||
| 375.04 |
0.58999 |
|
0.5899900 |
|
14.215 |
6.6801 |
|
||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
||||||||||
| Table. 4. Thermodynamic and
thermal properties of
propane (ρ, kg/m3) |
|
|
|
|
|||||
| |
|
|
|
|
|
|
|
||
| T, Ê |
P, MPa |
ρ' [14] |
ρ'' [14] |
ρ' [13] |
ρ'' [13] |
ÑV , J/(g
K) |
|
||
| |
|
|
|||||||
| [14] |
[13] |
|
|||||||
| |
|
|
|
|
|
|
|||
| |
|
|
|
|
|
|
|
|
|
| 270.15 |
4.427 |
539.9 |
|
|
|
1.627 |
|
|
|
| 270.67 |
– |
– |
|
531 |
|
– |
1.645 |
|
|
| 275.15 |
7.953 |
539.6 |
|
|
|
1.616 |
|
|
|
| 280.15 |
11.458 |
539.3 |
|
|
|
1.617 |
|
|
|
| 285.15 |
14.939 |
539.0 |
|
|
|
1.647 |
|
|
|
| 290.15 |
18.398 |
538.7 |
|
|
|
1.666 |
|
|
|
| 295.15 |
21.836 |
538.4 |
|
|
|
1.691 |
|
|
|
| 300.15 |
25.251 |
538.1 |
|
|
|
1.692 |
|
|
|
| 305.15 |
28.640 |
537.8 |
|
|
|
1.686 |
|
|
|
| 310.15 |
6.561 |
490.3 |
|
|
|
1.691 |
|
|
|
| 315.15 |
9.049 |
490.1 |
|
|
|
1.706 |
|
|
|
| 320.15 |
11.532 |
489.8 |
|
|
|
1.727 |
|
|
|
| 325.15 |
14.008 |
489.6 |
454.8 |
|
|
1.732 |
|
|
|
| 330.15 |
16.475 |
489.3 |
454.6 |
|
|
1.738 |
|
|
|
| 330.59 |
– |
– |
– |
436 |
|
– |
1.828 |
|
|
| 335.15 |
18.934 |
489.1 |
454.3 |
|
|
1.744 |
|
|
|
| 340.15 |
21.386 |
488.9 |
454.1 |
|
|
1.751 |
|
|
|
| 345.15 |
23.827 |
488.6 |
453.9 |
|
|
1.731 |
|
|
|
| 350.15 |
26.253 |
488.4 |
453.7 |
|
|
1.758 |
|
|
|
| 355.15 |
28.668 |
488.2 |
453.5 |
|
|
1.756 |
|
|
|
| 366.66 |
– |
– |
– |
306 |
|
– |
2.192 |
|
|
| 369.86 |
– |
– |
– |
229 |
|
– |
3.323 |
|
|
| Tcritical = 369.95 |
Ðêð = 4.200 |
ρcritic = 227 ± 2 |
|
|
|
|
|
|
|
| 369.88 |
– |
– |
|
|
224 |
– |
4.052 |
|
|
| 368.28 |
– |
– |
|
|
159 |
– |
2.903 |
|
|
| 375.15 |
5.095 |
301.1 |
|
|
|
2.236 |
|
|
|
| 380.15 |
5.744 |
301.0 |
|
|
|
2.110 |
|
|
|
| 385.15 |
6.404 |
300.9 |
|
|
|
2.032 |
|
|
|
| 390.15 |
7.072 |
300.8 |
|
|
|
1.952 |
|
|
|
| 395.15 |
7.746 |
300.7 |
|
|
|
1.924 |
|
|
|
| 400.15 |
8.425 |
300.6 |
|
|
|
1.824 |
|
|
|
| 405.15 |
9.109 |
300.5 |
|
|
|
1.788 |
|
|
|
| 410.15 |
9.795 |
300.4 |
|
|
|
1.814 |
|
|
|
| 420.15 |
11.176 |
300.2 |
|
|
|
1.810 |
|
|
|
| 425.15 |
11.869 |
300.1 |
|
|
|
1.854 |
|
|
|
| ÔÈÎ |
Äâîðÿí÷èêîâ Âàñèëèé
Èâàíîâè÷ |
| Íàçâàíèå ñòàòüè |
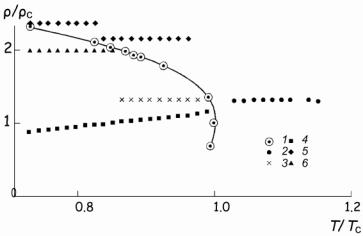
Isochoric Heat Capacity and T-ρ Dependence of
Cooling Agents along the Phase Equilibrium Line |
| Ðóáðèêà |
Òåõíè÷åñêèå
íàóêè. Õèìè÷åñêàÿ òåðìîäèíàìèêà. |
| Òåëåôîí (ñ êîäîì) |
89634115657 |
| E- mail |
vasiliy_dv01@mail.ru |
| Àäðåñ (äîìàøíèé) |
ã.Ìàõà÷êàëà,
óë. Êàðàáóäàãîâà, 7. |
| Èíäåêñ |
367000 |
Àëõàñîâ Àëèáåê Áàñèðîâè÷ – äîêòîð òåõ. íàóê, Èíñòèòóò ïðîáëåì ãåîòåðìèè, Äàãåñòàíñêèé íàó÷íûé öåíòð, Ðîññèéñêàÿ
àêàäåìèÿ íàóê.
Äâîðÿí÷èêîâ Âàñèëèé Èâàíîâè÷ – äîêòîð òåõ. íàóê, Èíñòèòóò ïðîáëåì ãåîòåðìèè, Äàãåñòàíñêèé íàó÷íûé öåíòð, Ðîññèéñêàÿ
àêàäåìèÿ íàóê. E-mail: vasiliy_dv01@mail.ru
Ðàáàäàíîâ Ãàäæè Àïïàñîâè÷ – êàíäèäàò
õèì. íàóê, Èíñòèòóò ïðîáëåì ãåîòåðìèè,
Äàãåñòàíñêèé íàó÷íûé öåíòð, Ðîññèéñêàÿ àêàäåìèÿ íàóê.
Ðàìàçàíîâà Äèíàðà
Ïàøàåâíà – àñïèðàíò,
Èíñòèòóò ïðîáëåì ãåîòåðìèè, Äàãåñòàíñêèé íàó÷íûé öåíòð, Ðîññèéñêàÿ
àêàäåìèÿ íàóê.
Alkhasov A. B. - Doctor of Technical Sciences, Institute
sor Geotheemal Problems of the Dagestan Scientific Centre of Russian Academy
of Sciences.
Dvoryanchicov V. I. – Doctor of Technical Sciences, Institute sor
Geotheemal Problems of the Dagestan Scientific Centre of Russian Academy
of Sciences.
Rabadanov G. A. – Candidate
of Chemical Sciences, Institute sor Geotheemal Problems of the
Dagestan Scientific Centre of Russian Academy of Sciences.
Ramazanova D. P. -
postgraduate, Institute sor Geotheemal
Problems of the Dagestan Scientific Centre of Russian Academy of Sciences.
Institute sor Geotheemal
Problems of the Dagestan Scientific Centre of Russian Academy of Sciences. Makhachkala, pr. Shamilya, 39a,
367030. Russian.