Ph.D., Ziyadullayev
O.E., masters Ergashev Yo.T.
Tashkent Chemical
Technological Institute, Uzbekistan
THEORETICAL
ABC OF GAMOGEN-CATALYTIC VINYLATION REACTION OF AROMATIC ACETYLENE ALCOHOLS
Recently,
scientific investigations on the creation of ecological safety and waste less
technologies, also, on the synthesis of
economically cheap chemical preparations with higher yield and to introduce them into the practice have
been carried out [1, 2].
Hence,
vinylation reaction on the basis of
aromatic acetylene alcohols (AAÀ) acetylene with higher base-catalytic system, The scheme of the
reaction has been offered as following [3, 4]:
![]()
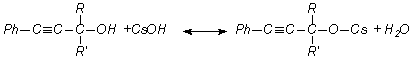
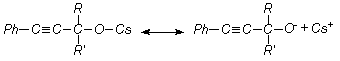
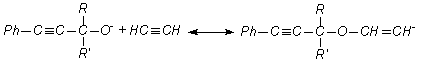
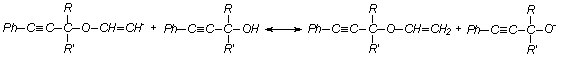
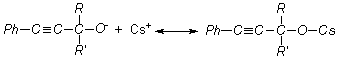
here;
R= -H, RI= -C6H5; R= -CH3, RI= -CH3; R= -CH3,
RI= -C2H5; R= -CH3,
RI= èçî -C3H7, R=
-CH3, RI= -C(ÑH3)3; R= -CH3,
RI= -Ñ6H3, Ph= C6H5.
Gamogen-catalytic
vinylation reaction on the basis of AAÀ MeOH-DMSO (MeOH- LiOH, NaOH and KOH) has been carried out and it was exposed that vinyl ethers (VE)
synthesized desirable in the interval of 40-60% [4].
It has been
observed the relatively maximum yield synthesis of AAÀ VE in the presence of
MeOH-CsF-DMSO with higher base-system on the experiment's results.
Table 1
The influence of nature and temperature of catalysts on the yield of AAÀ
VE
(duration of reaction is 6 hours, solvent DMSO)
|
Catalyst |
Temperature, oC |
I |
II |
III |
IV |
|
LiOH·CsF |
80 |
44,3 |
38,2 |
33,3 |
56,0 |
|
100 |
49,0 |
46,6 |
42,9 |
59,4 |
|
|
120 |
51,1 |
48,4 |
46,3 |
58,0 |
|
|
KOH·CsF |
80 |
49,6 |
45,2 |
40,2 |
57,4 |
|
100 |
57,4 |
52,0 |
40,7 |
66,4 |
|
|
120 |
58,3 |
55,7 |
49,6 |
67,3 |
|
|
NaOH·CsF |
80 |
74,2 |
73,2 |
67,3 |
79,6 |
|
100 |
84,8 |
77,1 |
72,3 |
88,0 |
|
|
120 |
86,0 |
79,7 |
75,0 |
89,5 |
here: I- VE 1-Fenil-3-metilpentin-1-ol-3;
II- VE 1-Fenil-3,4-dimetilpentin-1-ol-3;
III- VE
1-Fenil-3,4,4-trimetilpentin-1-ol-3;
IV- VE 1,3-Difenilbutin-1-ol-3.
The gained results show that as LiOH, NaOH and KOH are
used in the system of CsF-MOH-DMSO as MOH, in each particular state the yield of product goes through maximum while the temperature
is rising. It has been determined the increase of AAÀ on the series LiOH·CsF
< KOH·CsF < NaOH·CsF in the selected catalytic systems. As temperature
rose from 80 to 120 oC, the yield raised appreciably.
The selectivity of temperature 100 oC state
has been meant as the highest system for the process.
It can be explained that catalytic activity of
presented system with the formation of CsOH and NaF in the system and its less
solubility with the higher base-property of CsOH. In LiOH and KOH systems, the
solubility of the formed LiF and NaF is higher, and in the system they are
existed in the state of ion in the system, the balance specifies. Hereon, CsOH
couldn't perform completely activity. Owing to this LiOH+CsOH+LiF+CsF system is
in charge of catalyst.
It has been observed, vynilation process on the AAÀ
higher base-system, reaction undergoes under top level along with the formation
of polycomponent mixtures on the some stages. Herein, moving hydrogen of acetylene
goes on base of stereo-regioselectivity, also its exchanging process does
easily. Although investigations on the vinylation process of organic compounds
including members of various classes hydroxyl group containing in their
molecules have been carried out for years, reaction mechanism hasn't enough
researched scientifically yet. At present having formed catalytic active
center, reactions being carried out in the system MeO-CsF-DMSO, formation of
metal solvates and having become interval metal complex possessing active
center, counterbalances the function of catalyst.
As the result of investigation, it has been determined
that duration of reaction appreciably influences on the product yield.
Including when the process lasted for 6 hours, it was observed vinyl ethers
emerge with top yield in all selected systems.
When the process lasted 8 hours, it was observed the
yield of product decrease on the consequence of polycomponent mixtures
formation, dimerization of acetylene and polimerization of VE (Table 2).
Table
2
The
influence of nature and temperature of catalysts on the yield of AAÀ VE
(Temperature
100 oC, solvent DMSO)
|
Catalyst |
Duration of reaction, hour |
I |
II |
III |
IV |
|
LiOH·CsF |
4 |
43,8 |
39,0 |
36,8 |
54,8 |
|
6 |
49,0 |
46,6 |
42,9 |
59,4 |
|
|
8 |
42,4 |
37,8 |
34,1 |
52,2 |
|
|
KOH·CsF |
4 |
48,4 |
46,3 |
44,4 |
62,0 |
|
6 |
57,4 |
52,0 |
47,0 |
66,4 |
|
|
8 |
47,2 |
42,2 |
40,1 |
58,4 |
|
|
NaOH·CsF |
4 |
78,2 |
73,0 |
67,7 |
79,8 |
|
6 |
84,8 |
77,1 |
72,3 |
88,0 |
|
|
8 |
73,4 |
70,8 |
65,5 |
76,3 |
It has been
determined that the yield of product emerge at very maximum: I=84,8%; II=77,1%;
III=72,3% and IV=88,0% in the interval of catalysts LiOH·CsF, KOH·CsF as
NaOH·CsF is used. We can explain the exact higher yield of VE quantity in
NaOH·CsF state than in KOH·CsF state as stated below:
- as the result
of fusing of sodium hydroxide in DMSO solution, exchanging reaction with CsF
goes to the end , forming CsOH quantity relatively more, forms metal complex
possessed active catalytic center and it just reacts with acetylene easily;
- new higher
state is observed in potassium
hydroxide too, but formation of potassium and cesium alcoholates two kinds of catalysate have been formed in
the system of AAÀ active centre,
in the account of being difficult to separate them from each other, quantity of
additional products form relatively more, and this demands extra product
alongside with complication of acetylene combination. As a result, in order to
increase the yield of product it leads to continue the process longer, to use
extra acetylene and solvent, to increase economical expense. For these reasons
the cost of VE rises in price;
- Because of being higher of the solubility degree of KF than NaF, having
formed interval compound with DMSO, KF causes quantity of CsOH to be less.
Conclusion, in order to synthesize AAÀ VE with higher yield - 6 hour's process at
100 oC temperature in the system of NaOH-CsF-DMSO has been chosen.
In this case it has been determined that very maximum yield of product is
I=84,8%; II=77,1%; III=72,3% and IV=88,0%.
Literature:
1. Temkin O. N. Acetylene chemistry: “Acetylene tree” in
organic synthesis on the eve of xxi century // Journal Sorosov of science. Ò.7., ¹6, 2001. p. 32-46.
2. Schelkunov S.À., Sivolova À.Î., Ìàtàåvà S.Î.,
Ìinbaev D.B., Ìuldakhmedov Z.Ì. // Journal of organic chemistry. 2001.
Ò.37. V. 1. p. 17-20.
3. Belov B.I., Êozlov V.V. // Success of
chemistry, 1963. Is. 32. ¹ 2. p. 121-153.
4. Yunusov
R.Yu. Organic chemistry. Òàshkent.: Uzbekistan, 1995.
V. 1. p. 725.
5. Ziyadullaev O.E. Synthesis and technological of aromatic acetylenic alcohols, their vinyl
ethers on the base of phenylacetylene:
des. cand. chem. sc. Tashkent. 2011. p. 213.