UDC
622.343.9
NEW CYANIDE FREE TECHNOLOGY FOR ELECTROCHEMICAL
EXTRACTION OF GOLD FROM SULFIDE ORES
Candidate of technical sciences, senior researcher,
associate professor Oryngojin Ye. S.
Kazakhstan,
Almaty
Mining
Institute after D.A.Kunaev
Development of gold-mining industry of Kazakhstan is connected with the
insufficient involvement of sulfide ores in mining and processing, which are
complex in composition and persistent in gold extraction. Technologies of
sulfide ores processing applied at Kazakhstan plants are distinguished with
high material W labor costs; they are multi-operational and not environmentally
safe. Therefore, search and creation of new effective technologies for
gold-containing ore processing is exceptionally important economic assignment.
With the view of solution of this problem, the National Engineering
Academy of the Republic of Kazakhstan has developed new electrochemical,
cyanide free technology for gold leaching from persistent sulfide-containing
raw material. The technology has successfully passed a stage of semi-industrial
tests resulting in the production of gold in the solution by means of the plant
manufactured in Stepnogorsk city. The next stage of development was the
elaboration of technology for gold extraction from sulfide-containing raw
material by combining the processes of electrochemical leaching and sorption in
one technological device.
The idea of combination included the creation of U-shaped
electrochemical reactor, in which one branch is separated for cathode and anode
chambers with interelectrode cation-exchange partition, and the other branch of
the device is designed for airlift mixing of the ore grinded till the class
0,074 mm in 20-30% sodium chloride solution. At that the process of
electrochemical gold leaching and sorption is combined with the gold
concentration on the cathode covered with coal fiber.
To implement the idea, the flowchart and the chart of its hardware
design have been developed. Hardware chart including two consequently working
U-shaped devices for electrochemical sorption gold leaching is shown in the
picture 1.
Beforehand, the studies by means of devices of box type have been
realized.
The operation of devices of electrochemical leaching of box type with
the use of cation-exchange membranes for the separation of cathode and anode
chambers revealed the opportunity of their application, but in short-term
operation – in pulp environments the membranes turned out to be short-lived and
in several cycles showed cracks in the membrane MKK-1.
The gold came to the cationite into the cathode chamber separated from
the anode chamber with the ion-exchange membrane only in the form of anion
complex and the process rate was limited by the ion diffusion rate through the
cation-exchange membrane that led to the increase of leaching time. The pulp
after the electric treatment was discharged from the anode chamber, i.e. from
the place where gold proper transformed into solution that stipulated the
multi-stage process of the electrochemical leaching.
Based on the experienced gained, basically new structure of
interelectrode partition was developed. The partition consists of two corrosion
plates, in each of which holes are drilled angularly. Hydrogen extracted at
cathode, chlorine at anode, as a result of electrochemical reaction, in this
case cannot arbitrarily mix with each other any more, since the inclined holes
result in the limited access to the zone of opposite sign; recovery of oxidized
gold with hydrogen is minimized. At the same time, relatively free access for
the pulp from one chamber to another is ensured, that leads to involvement of
the whole volume of the pulp in the electrochemical process and helps to
discharge the de-gilded pulp from any chamber.
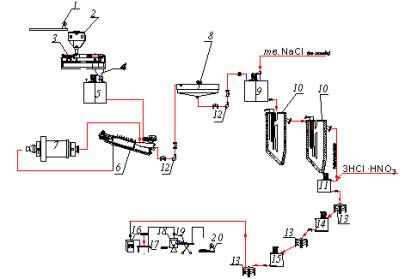
1- scales; 2 - bunker; 3 - auger feeder; 4 - chute;
5 - conditioner; 6 - sizing screen; 7 - mill; 8 - thickener; 9 - repulper; 10 –
device for electrosorption leaching; 11 - reactor; 12 - pump; 13 - nutsch
filter; 14 – settling tank; 15 - arrester; 16 – tempering cabinet; 17 -
burdening table; 18 – gold-melting oven; 19 - mould; 20 – laboratory scales
Picture 1 - Hardware chart of electrochemical,
sorption gold leaching
As an anode, graphite was used, as a cathode, coal-fiber material based
on graphite with the cover made of filter fabric. Such structure of cathode was
explained by the need to regularly regenerate it. The cover made of filter
fabric ensuring free access for solute gold to a cathode hinders the ingress of
particles of ore material that significantly facilitates the process of regeneration
of coal fiber.
The first test pachuca for electrochemical leaching was remade in
conformity with the idea described above; cathode and anode chambers were
equipped with new partitions and established at one branch of the U-shaped
device, and the second branch served for the airlift mixing of the pulp. The
total volume of both branches of the device is – 200 dm3.
The second 250 dm3 device was made of polyethylene pipe, 330
mm in diameter. The laboratory tests proved the efficiency of use of coal fiber
as a cathode that allowed the simplification of the gold extraction flowchart.
The process of ion exchange gold extraction for ionite resins with numerous
auxiliary operations for resin cleaning from the sludge and regenerating
solution was need no more. There was no need in the assembly for preparation of
desorption solution, in the use of sizing screens to separate the resin before
and after desorption etc.
To carry out the tests, sulfide raw material containing 11,0 g/t of gold
was used – tails of gravity concentration of sulfide ores of “Altyn Alyans – 3”
Ltd in amount of 2 t.
Semi-industrial test of the new technology was carried out in the
following regime:
Voltage – 4,2 – 4,6 v;
Anode current density – 780-820 à/m2;
Pulp density – 1,35 kg/dm3;
S:L Relation = 1 : 3;
NaCl concentration – 200 – 220 g/dm3;
Duration of electrochemical treatment is 6 – 8 hours.
During the test, the value pH
was noted at the output of pulp. Pulp sampling was taken every hour and was
averaged for 8 hours; the averaged sample was analyzed for gold content.
As a coal cathode, coal fiber of the class ÍÒÌ –
100 was used. The current was supplied to the coal fiber through the graphite
framework on which the coal fiber was coiled and then glass fiber cloth.
Consumption of coal fiber per every cathode was 20 g. The results of the tests
are given in the table 1. They show that: concentration of gold in liquid phase
of waste pulp at the output from the second pachuca is 0,02–0,03 mg/dm3,
that corresponds to 99,5% of its extraction. The results of the tests showed
that involvement of one or two additional pachucas in the process flowchart
ensures an opportunity to minimize the loss of gold in the liquid phase.
During the first series of tests at the duration of electrotreatment of
8 hours, the residual content of gold cleansed cakes amounted to ~ 1,7 g/t,
that corresponds to 84% of gold extraction. Reduction of process time till 5
hours results in reduction of gold extraction by 9-10%.
The use in the tests relatively poor raw material did not allow the
achievement of maximum capacity of electrocoal – the coal cathode with the
capacity of 22 g/kg of gold was separated for regeneration. The rest of the
gold was most probably left as a material in process.
Coal cathode was washed with water to cleanse from sludge, was
dismantled from the graphite framework and treated with aqua regia at S:L
relation = 1:5. After this treatment the coal fiber was washed with water, the
wash water was mixed with gold-containing eluate, diluted with water by ~1,5 –
2 times. After the solution was settled during one hour, the sediment of the
liquid phase was separated by means of the filtering. Out of the filtrate, the
coffee like gold-containing sediment was extracted by means of treatment with
the sodium sulfite; and then by means of melting 0,35 g of gold was produced.
The analysis of the remnants of the coal fiber after its treatment with
aqua regia and washing with water showed that they contained up to 8 g/kg of
gold. Relatively high residual capacitance of coal fiber proves the
advisability of its direct treatment by means of burning and consequent
extraction of gold from the ash, since the operation of cleansing of coal fiber
from the sludge is quite labor intensive.
In whole, generalizing the results of the research of the developed
technology, it is possible to state that the chart of combination of operations
of electrochemical leaching and electrosorption with the use of coal fiber
material is quite effective and operative and after certain upgrade can be
recommended for implementation in industrial practice of gold production.