Omorov T.M.
DIAGNOSIS AND TREATMENT OF CEREBRAL ECHINOCOCCOSIS
INTRODUCTION
Echinococcosis is a widely as distributed
disease of low prevalence. The problems are not so much the numbers of
patients, but the fact is hat the disease is highly pathogenic in affected
patients. In the literature, between 43%-66% of cases present as liver disease,
32%-37% as pulmonary disease, 13,6% as concurrent pulmonary and liver disease,
with 0,2% involving the lungs, liver and brain. (Akmatov 1994, Akshulakov et al
2000, Akhunbaev 1964, Kornyansky et al 1968, Petrovsky et al, Rosin 1991,
Jimenes-Mejias et al 1991).
The prognosis for patients with
echinococcosis depends on early and accurate diagnosis and prompt surgical or
chemotherapeutic treatment. Whilst the problem of diagnosis in many organ
systems has been solved, diagnosis of cerebral echinococcosis still represents
a significant challenge. Frequently cerebral echinococcosis is misdiagnosed for
other conditions such as tumours, abscesses or not parasitic cysts (Kariev et
al 1990, Junarddi et al 1990). Late diagnosis of cerebral echinococcosis often
leads to a poor prognosis. There are frequent complications during Suring to
remove cerebral cysts. Rupture of the cysts can lead to an outpouring of
contents that include germinal elements, pus and necrotic elements, into the
serous cavities of patients. This can lead to serious complications such as
allergic reactions, anaphylactic shock, epileptic attacks, suppuration and
recurrence of disease (Akmatov et al 1994, Akshunbaev et al 2000, Kariev et al
1990, Petrovsky et al 1985, Jimenes-Mejias et al 1991, Kayu et al 1975). In
addition, symptoms may worsen and central nervous system encephalitis may
develop, intravascular coagulation and other problems associated with the disintegration
of the cyst and the leakage of cyst fluid which contains acetic and lactic acid
and other substances, into the blood. Serotonin, prostaglandin and kinins may
be released in response this release of cyst fluid (Ersahim et al 1993). This
can result in cerebral hypostasis and brain swelling (Akhunbaev 1964, Petrovsky
et al 1985, Lunarddi et al 1990). Damage to the ventricular systems can alter
the fluid dynamics in the brain, leading to pressure on aub-arachnoid tissues
and angiospasm.
Epilepsy is a frequent complication of
cerebral echinococcosis as cyst fluid is allergenic and irritant to the brain
tissue and protoscolices are sometimes not completely removed leading to
daughter cysts and relapses.
The underlying reasons for these pathogenic
mechanisms is the space occupying lesion and the leakage of blood and necrotic
or cyst material into the sub-arachnoid space. The important phase of the
treatment with cerebral echinococcosis, after removal of the cysts, is adequate
drainage of nercotic material and blood from the sub-arachnoid spaces. To
remove blood, passive drainage using a polyethylene or rubber tube is required.
However, this drainage requires close supervision as the tubes are frequently
blocked with blood clots or detritus. Even wide bore tubes need constant supervision. A technique for the drainage
of fluid from the brain has been devised. Blood and other detritus are flushed
out of the sub-archnoid spaces by perfusion fluids. However, the technique has
to be undertaken with great care to avoid increasing intracranial pressure or
bleeding.
The purpose of this study was to define the
clinical characteristics of cerebral echinococcosis, the rate of parasite
growth and the topographical relationships with cerebral white and grey matter
and the ventricular system, and then to study the active draining of the
sub-arachnoid spaces following cyst removal.
MATERIALS AND METHODS
In the last 15 years, 105 patients have presented with
cerebral echinococcosis. This represents 0.9 % of
all patients treated with space occupying lesions of the brain.
In a total of 65 patients external drainage from the
cranial cavity was undertaken. In these patients the efficacy was assessed
by changes in intra-cranial pressure and cerebral circulation by
impedance radiography.
RESULTS AND DISCUSSION
Initial symptoms often appeared long before
clinical diagnosis. A space occupying lesion was suspected based on evolving central and
peripheral neurological symptoms. In 38 patients, cerebral echinococcosis
was confirmed with the application of computer and nuclear magnetic resonance tomography. In all these patients
neurological symptoms like exophthalmoses, paresis, titanic spasms
of the neck muscles, horizontal nystagmus with rotary components,
and problems of coordination were
marked. In 12 patients the first symptoms observed were clonic epileptiform attacks up to 10 years before diagnosis.
There developed into focal epileptiform attacks and localized
pyramidal symptoms. In 43 patients the first symptoms were
headaches. In 17 of these patients headaches
occurred only a few months before diagnosis although this was many years after the first symptoms (such as epileptiform
seizures). In 9 patients, headache was the presenting clinical
symptom.
Marked eosinophilia was seen in 53 of the
65 patients. Radiological evidence of the development
of cerebal hypertension includes an increase in the size of the
cranium, impressions on the base of the
cranium, funnel finger shaped depression, hypertrophy of ventricular veins and rarefaction of the skull. The radiological
changes depend on the sizes of the hydatid cysts and disease
duration. Intensity of headaches did not correlate with the
hypertensive changes of the cranium.
Congestion
of the ocular fundus
was seen in 63 patients. In 11 of then, these had developed into
secondary atrophy of the optic nerves. Development of this
syndrome is often associated with massive intracranial
pressure and obstruction of drainage from the ventricular and
paraventricular systems by cysts with a volume of
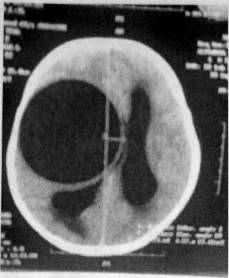
200-250 cm3 The size of cysts from this series of patients varied between 13cm and 12cm in diameter.
Cerebral spinal fluid of 38
patients was normal and in 4 patients there was a moderate increase
in lymphocytes.
According to the computer and nuclear magnetic
tomography, the cysts are located in the white matter, mainly in the temporal
occipital lobe (Ersahin et al 1993). Of 65 patients presenting with cerebral
echinococcosis, 53 had single cyts, whilst 4 also had pulmaonary cysts and 4
had cyts in multiple locations. Single cysts were seen in the white matter of
the frontal, parietal and temporal lobes and in the ventricular cavity.
Patients with pulmonary cysts had 2*5 cerebral cysts in the white matter on the
convex and basal loves of the brain. The severity of clinical signs was often
not associated with the size but number and location of lesions. We compared
the spontaneous chemiluminescence with composition of the protein in the
cerebral spinal fluid . Weak fluorescence does not depend on (he quantity
but type of protein present. When biological liquids are exposed
to ultraviolet lights reactions of free radicals
can lead to the formation of unidentified fatty acids. Thus the
intensity of photo-induced chemiluminescence depend on processes
at the cellular level and on the level of exposure.(pic I).
In a series od cases of cerebrai echinococcosis and
controls we have investigated the magnitude of chemiluminesce of the CSF.
Comparison of the data suggests that CSF from cases with cerebral echinococcosis
have more intense chemiluminescence than controls; this is an additional
criterion that can be used in assessing a diagnosis.
When there is intracranial haemorrhage good drainage
is required in 3 circumstances. In the first, which was encountered in 14
patients, good perfusion and drainage of the subarachnoid space and areas of
surgical intervention were provided. Such conditions occur 2-3 hours after
surgery. For rapid removal of the intracranial fluid, rapid perfusion is
required with the drainage tube below the head of the patient. If the drainage
tube is blocked, even partially, then there can be an uncontrollable increase
in intracranial pressure with hypertensive syndromes developing.
The second situation arises when the outflow of fluid
is greater then the inflow during perfusion drainage caused by the siphon, and
thus creating negative intracranial pressure. This negative intracranial
pressure can lead to haemorrhage. In 29 patients full haemostasis had been
achieved within 30-60 minutes postoperatively as shown by the absence of blood
from the perfusion tubes. The perfusion flow was decreased but in a number of
patients haemorrhage commenced again within 20 minutes. This was controlled in
12 patients by increasing the perfusion rate again and adjusting the pressure
of the drainage fluid. In 7 patients, haemostatic medical therapy was required.
In 2 patients the surgical site was reopened and haemotoma was removed.
In the third situation complete or partial blockage of
the drainage tubes by blood clots or brain detritus can lead to a rapid
increase in intracranial pressure. Increase in intcacranial pressure was
observed in 22 patients who clinically presented with intense headache, nausea,
vomiting, shivering, psychomotor anxiety and excitation. Loss of consciousness,
tachypnoea and tachycardia occurred in 5 patients. After emergency intervention
to remove the excess fluid, the patients rapidly relopsed in to normaky.
Usually, even in the presence of such adverse
pathological problems, providing adequate drainage is provided for 2 days, few
long-term effects are witnessed in the majority of patients.
During non-con trolled perfusion drainage
under negative intracranial pressure, fluctuations of the venous tension are
not seen. Pulsatory and respiratory fluctuations on plethysmogram curves are
not defined.
Only aperiodic changes in pressure are
reflected by the bodily position. Gradual increases in intracranial
pressure begin some 5-6 hours after surgery with an increase in the amplitudes
of pressure changes.(pic 2).
|
Pic 2. |
The long-tern follow-up of patients, who were given active intracranial perfusion, were investigated. In the first two groups, there were no relapses; in the third were 2 relapses out of 22 of interventions.
Good drainage of the intracranial cavity
after sugery provides optimum conditions for the removal of debris (such as from the cyst), blood and
necrotic material. This permits the establishment of normal intracranial
pressure, blood circulation and brain metabolism. The condition of the patient
improves, consciousness is restored, and clinical symptomatology decreases.
Meningeal and fever responses do not develop, or only moderately, and then
disappear within 2 days. There are decreased signs of thirst, headache,
vomiting, psychomotor anxiety and excitement. Blood pressure is stabilized and
respiratory and pulse rates return to normal.
Thus, adjustable perfusion-drainage of the
cranial cavity is an important and effective postoperative treatment for
cerebral echinococcosis. The patients condition is improved in the postoperative
period and the time spent in hospital is reduced.
CONCLUSION
Based on our surgical experience, and
others reported in the literature, the important features in die diagnosis and
management of cerebral echinococcosis include: 1 )a history of episodic pyrexia
of unknown origin 2)the presence of shot-term serous meningitis and epileptic
attacks 3)slowly progressing focal neurological disease discrepancies between
changes in the radiological appearance of bones of the cranium and duration of
clinical neurological signs 5)data from computer tomography and magnetic
resonance imaging in defining the number, size and location of cerebral cysts
and any pathological changes in surrounding cerebral tissue 6)chemiluminescence
of CSF 7)adjustablc perfusion drainage of the cranial cavity following removal
of the cyst enables supervision of intracranial pressure during the post
operative period 8)flushing out of protoscolices as a result of cyst rupture
reduces the rick of recurrence.
References
1.
AKMATOV В A (1994) Echinococcosis. Bishkek,
pp 6-131
2.
AKSHULAKOV
SK, HACHATRYN VA&MAKHAMBETOV ET (2000) Echinococcosis of the central
nervous system. Almaty, 23 pp.
3.
AKHUNBAEV IK
(1964) Echinococcosis. Great Soviet Encyclopedia, 35, 885-895. CATALTEPE et al
(1992) Intracranial hydatid cysts: experience with surgical treatment of 120
patients.
4.
Neurochirurgia
35, 108-111.
5.
ERSAHIN Y, MULTLER & CUZELBAB (1993) Intracranial
hydatid cysts in children. Neurosurgery 332, 219-224/
6.
MENES-MELAS ME et al (1991) Hidatidosis cerebral. Med
Clin (Barcelona) 97,125-132. KFR1EV MN, AZAROVA TG, MALAMUT et al (1990) Actual
questions of alveococcosis. Tashkent pp 84-85.
7.
KAY A U et al (1975) Intracranial hydatid cysts. Study
of 17 cases. Journal of Neurosurgery 42, 580-584.
8.
KORNYASKY GP, BASIN & EPSHTEIN IV (1968) Parasitic
diseases of the central nervous system. Moscow, pp 79-139.
9.
LUNARDDI P et al (1990) Cerebral hydatidosis in
childhood. Neurosurgery 36, 312-314/ PETROVSKY BV, MILONOV OB & DESNICHIN P
(1985). Surgery of echinococcisis . Moscow.
10.
ROSIN VS (1991) Diagnosis of cystic echinococcisis of
the brain. Contemporary Medicine 2, 84-86.
