Strusovskaya O.G., Artemyeva A.P., Afonina
S.A.
The Northern State
Medical University, Arkhangelsk, Russia
PHENOLIC COMPOUNDS of COCHLEARIA OFFICINALIS, GROWING
ON THE SOLOVETSKY ARCHIPELAGO
Cochlearia
officinalis belongs to the Cruciferous family (Crucifereae L.) and is a
wide-spread plant in the European North. Cochlearia officinalis has long been used
in traditional medicine as an antiscorbutic drug and also in treating
gastro-intestinal diseases. However, the chemical composition of the plant
has not been sufficiently studied.
The
purpose of this study was to determine the polyphenolic substances of
Cochlearia officinalis. The subject of the research was air-dried raw material
plants of second growing year, harvested during flowering period in summer 2011
on the islands of the Solovetsky archipelago and dried by air-cured in a well
ventilated area at 22-24°C. Preliminary analysis of the qualitative
composition of phenolic compounds was based on the method of one-dimensional
chromatography in a thin layer of sorbent (TLC). For this purpose,
air-dried raw material of Cochlearia officinalis was crashed and sieved through
laboratory sieves S30/50 (State standart (GOST) 3826-82) with an aperture size
of 1 mm. Ethyl alcohol 70% was used as an extractant (State standart
(GOST) 51652-2000). The exact mass
of crushed plant material (about 1.0 g) was placed in a round bottom flask from
a heat-resistant glass with ground joint, then 10 ml of alcohol was added, and
the flask was attached to a reflux condenser and placed on a boiling water
bath. The heating was performed for 45 min. The resulting alcoholic
extract was cooled, filtered through a blue ribbon filter (Technological
conditions 11.3.03) and evaporated to a volume of approximately 0.5 ml. In order to select solvents system for optimal separating components of
the extract, the following mixtures were used: chloroform-ethyl alcohol-water
(26:14-3) [1], n-butanol-acetic acid-water (BAW) (4:1:5); BAW (4:1:2) [2]
BAW (5:1:1) [3]. The resulting extract (10 μl) and 10 μl of 1% solutions of tracking substances (routine (Sigma-Aldrich
207671-50-9), quercetin (Sigma-Aldrich 117-39-5), kaempferol (Sigma-Aldrich
520-18-3 ) quercitin (Fluka 522-12-3); isoquercitin (Fluka 482-35-9);
hyperoside (Sigma-Aldrich 482-36-0) and luteolin (Sigma-Aldrich 491-70-3)) were
spread on the starting line of the plate «Silica gel 60 F254»
(Merk). The
plate then was placed in a chromatographic chamber, which had previously been
saturated with eluent vapor for 6 hours. Chromatogram was made in the
ascending manner. When the eluent front
reached 10 cm, the plate was removed from the chromatographic chamber, air
dried and viewed in the visible and ultraviolet light at a wavelength of 254 nm
before and after treating the chromatograms with alcoholic solution 5% of
aluminum chloride [4].
During
the studies it was found out that the most complete separation of the
components of the mixture is observed in the solvent system BAW (4:1:5). Under the selected
conditions, on the chromatogram of alcoholic extract of Cochlearia officinalis
four spots were detected with the values of Rf = 0,17; 0,43; 0,68
and 0.90. The spots with the values of Rf = 0,43; 0,68 and
0.90 corresponded to the values of Rf of the spots of
standard samples of rutin, hyperoside, and quercetin, respectively. The
spot corresponding to the routine was dominant one. To identify the spot
with the value of Rf = 0,17, the chlorogenic acid (Sigma-Aldrich 327-97-9) and
tannin (Sigma-Aldrich 1401-55-4) were used as tracking substances. In the
course of the studies it was found that the spot with the value of Rf = 0,17
corresponded to chlorogenic acid.
Amount
of flavonoids in the material studied was determined by the method of
spectrophotometry, by reaction with the solution of aluminum chloride [1].
Optimal
conditions for the quantitative analysis were selected experimentally by
varying concentration of the eluent, the time of extraction, concentration and
the amount of added solutions of aluminum chloride 2% and acetic acid, exposure
time after adding of reagent solutions.
In
the course of the research, a methodology was developed for determining the
quantitative content of flavonoids in the tested plant materials: about 1.0 g
(exact mass) of crushed (to a size of 1 mm) air-dried raw material of
Cochlearia officinalis is placed in a round bottom flask from heat-resistant
glass with ground joint, then 50 ml of ethyl alcohol 70% is added. The flask is attached to
a reflux condenser and placed in a boiling water bath. The heating is
carried out for 15 min, the extraction obtained is filtered through a paper
filter into a volumetric flask with a capacity of 100 ml, preventing ingress of
raw material particles to the filter. The extraction is carried out three
times. The volume of the combined extracts is adjusted in a volumetric
flask with ethyl alcohol 70% to the mark (solution A).
1
ml of solution A is placed in a flask with a capacity of 25 ml, 2 ml of 2%
solution of aluminum chloride in ethyl
alcohol 95%, 0.1 ml of diluted acetic acid is added, the volume of the
resulting solution is adjusted with 95% ethyl alcohol to the mark (solution
B). After
40 min, in quartz cells with thickness of layer 1 cm, the optical density of
the solution is measured relative to the reference solution which is solution B
without adding the solution of aluminum chloride. The reference solution of
rutin 0.05% (RIS) prepared similarly to that under study is used as the
standard. Differential spectra of the investigated and standard solutions
are presented in Fig. 1.
The
quantitative content of flavonoids content (X%) in air-dried raw material
Cochlearia officinalis is calculated using the formula:
![]()
![]()
![]()
![]()
A – optical density of the solution under study;
As- optical density of the routine reference solution;
a – weigh of raw material, g;
as –weigh of standard routine sample, g;
b - moisture level in raw material,%.
The
results of determination of the quantitative content of flavonoids in air-dried
raw material of Cochlearia officinalis are presented in Table 1.
During
the studies of the UV absorption spectrum of the alcoholic extract of air-dried
raw material of Cochlearia officinalis, it was found out that the maximum
optical density is observed at a wavelength of 270 nm ± 2 nm. Gallic acid
0.001% solution has the similar maximum of optical density (Fig. 2).
Quantitative
determination of the amount of phenol carbonic acids was performed by direct UV
spectrophotometry, using as a reference solution alcohol solution 0.001% of
gallic acid [5]. For
this purpose, approximately 1 g (exact mass) of crushed (to a particle size of
1 mm) air-dried raw Cochlearia officinalis was placed in a flask from a
heat-resistant glass with ground joint with a capacity of 100 ml, then 50 ml of
ethyl alcohol 70% was added. The flask was attached to a reflux condenser
and heated on a water bath for 1 hour. After cooling, the extract was
filtered into a volumetric flask of 100 ml. The process was
repeated. The combined extract was adjusted with ethyl alcohol 70% to the
mark (solution A).
5
ml of solution A was placed in a flask with a capacity of 100 ml, volume of
solution in the flask was adjusted with ethyl alcohol 70% to the mark (solution
B). Optical
density of solution B was measured by a spectrophotometer in a cuvette with a
layer thickness of 10 mm, using ethyl alcohol 70% as a reference solution.
The optical density of the reference solution of gallic acid was measured
simultaneously. To prepare the reference solution, about 0.05 g of gallic
acid was placed in a flask with a capacity of 100 ml, 30 ml of ethyl alcohol
70% was added and the mixture was stirred until complete dissolution. The
amount of solution in the flask was adjusted with the same solvent to the
mark. 2 ml of the resulting solution was transferred into a volumetric
flask of 100 ml. The amount of solution
in the flask was adjusted to the mark with ethyl alcohol 70%.
The
content of the sum of phenol carbonic acids (%) in terms of gallic acid and
absolutely dry raw material was carried out using the formula:
![]()
Dx
- optical density of the
solution;
D0 - optical density of the reference solution of gallic acid;
as - weight of gallic acid, g;
a - weight of air-dried raw Cochlearia officinalis, g
b - loss in weight when drying raw material,%.
The
results of the quantitative determination of the amount of phenol carbonic acid
in air-dry raw material Cochlearia officinalis are presented in Table 1.
Since
the chromatogram of alcoholic extract of raw material of Cochlearia officinalis
had a spot corresponding to chlorogenic acid, and during the study of the UV
spectrum the maximum optical density was observed at a wavelength of 330 nm ± 2
nm (Fig. 4), which is the characteristic of the alcoholic solution of this
substance, the amount of phenylpropanoids in the material under study was
determined by direct UV spectrophotometry in terms of chlorogenic acid [6]. For that, about 1.0 g
(exact mass) of air-dried raw material of Cochlearia officinalis was crushed
(to a particle size of 1 mm) and placed in a flask from a heat-resistant glass
with a ground joint with a capacity of 100 ml, then 50 ml of ethyl alcohol 70% was added. The flask
was attached to a reflux condenser and heated on a boiling water bath for 1
hour. After cooling, the extract was filtered into a
volumetric flask 100 ml through a blue ribbon filter and the volume of the
solution in the flask was adjusted with ethyl alcohol 70% to the mark (solution
A).
5
ml of solution A was placed in a flask with a capacity of 100 ml, volume of the
solution in the flask was adjusted with ethyl alcohol 70% to the mark (solution
B). Optical
density of solution B was measured by a spectrophotometer in a cell with a
layer thickness of 10 mm, using ethyl alcohol 70% as a reference solution.
The optical density of the reference solution of chlorogenic acid was measured
simultaneously (Fig. 3). To prepare the reference solution, about 0.05 g
of chlorogenic acid was placed in a flask with a capacity of 100 ml, then 30 ml
of 70% ethyl alcohol was added, and the mixture was stirred until complete
dissolution. The amount of the solution in the flask was adjusted with the
same solvent to the mark. 2 ml of the resulting solution was transferred
into a volumetric flask of 100 ml. The amount of the solution in the flask
was adjusted with ethyl alcohol 70% to the mark.
The content of phenylpropanoids (%) in terms of chlorogenic acid and absolutely
dry raw material was calculated using the formula:
![]()
Dx
- optical density of the
solution;
D0 - optical density of the reference solution of gallic acid;
as - weight of gallic acid, g;
a - weight of air-dried raw Cochlearia officinalis, g
b - loss in weight when drying raw material,%.
The
results of the quantitative determination of the amount of phenylpropanoids in
air-dry raw material of Cochlearia officinalis are presented in Table 1.
Thus,
in the course of study it has been found out that air-dry raw material of
Cochlearia officinalis contains chlorogenic acid and the following flavonoids:
rutin, quercetin, and hyperoside, with routine as the dominant component. The quantitative content
of biologically active substances in the studied materials was as follows: the
amount of flavonoids - 0.66%, the amount of phenol carbonic acids - 1.62%, the
amount of phenylpropanoids in terms of chlorogenic acid - 1.54%.
References:
1. Anisimova
M.M. Qualitative
and quantitative analysis of flavonoids in buckwheat herb / M.M. Anisimova,
V.A. Kurkin,
V.N. Yezhkov
// Proceedings of the Samara scientific centre of the Russian Academy
of Sciences. - 2010. - V.12 .- № 1(8) .- P. 2011-2014.
2. Lobanov
I.Yu. Extracting
and study of the flavonoids of leaves of aspen pine / I.Yu. Lobanova, V.F. Turetskova
// Chemistry of plant raw material. - 2011. – No. 2. - P. 117-122.
3. Beketov
E.V. Identification
and quantification of flavonoids in the fruits of bird cherry // E.V. Beketov,
A.A. Abramov,
O.V. Nesterova, S.V. Kondrashov
// Vestnik of
the Moscow University. ser.
2. Chemistry.-2005. - V. 46. – No. 4 .- P. 259-262.
4. Fedoseeva
L.M. Study
of flavonoids of red leaves of bergenia tolstolistnogo (Bergenia Crassifolia
(L.) Fitsch. growing in the Altai / L.M. Fedoseeva, E.V. Timokhin // Chemistry
of plant raw material. - 1999. – No. 4. P. 81 - 84.
5. Gavrilin
M.V. Phenolic
compounds of above-ground parts of clary sage (Salvia sclarea L.) cultivated in
the Stavropol Territory // M.V. Gavrilin, O.I. Popova,
E.A. Gubanov
// Chemistry of plant raw material.- 2010 .- No. 4 .- P. 99-104.
6. Olennikov
D.N. Phenolic
compounds of leaves of Sacalia hastata. L. and their quantitative analysis //
D.N. Olennikov, L.M. Tanhaeva
// Chemistry of plant raw material. - 2011. – No.3. - P. 143-148.
Fig. 1
Differential UV spectra of solutions
of the studied substances
1 2
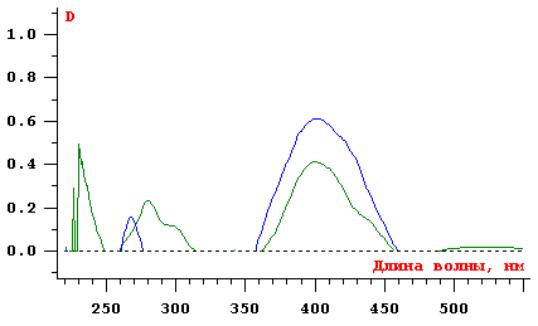
1- UV spectrum of the 0.05% solution of the reference
solution of routine;
2- UV spectrum of the
complex alcoholic extract of Cochlearia officinalis
Fig.2
UV spectrum of the
complex alcoholic extract of Cochlearia officinalis
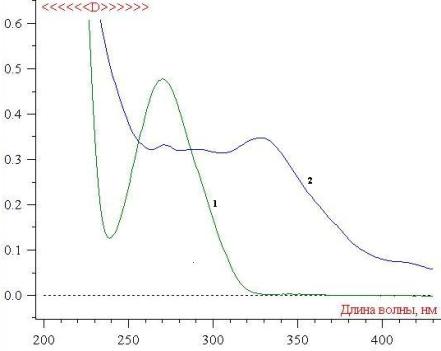
1
- 0.001% alcoholic solution of gallic acid;
2
- Alcoholic extract of air-dried raw material of Cochlearia
officinalis
Fig.3
UV-spectra of optical density
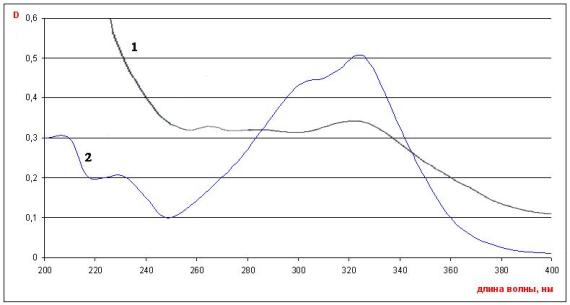
1-alcoholic extract of air-dried
raw material of Cochlearia officinalis;
2-0,001% alcoholic solution
of chlorogenic acid
Table
1
The
results of the quantitative determination of the phenolic compounds in
air-dried raw
material of Cochlearia officinalis
|
Determinate
rate |
|
|
|
ε, % |
|
The
amount of flavonoids |
0,658 |
0,012 |
0,031 |
±4,78 |
|
The
amount of phenolcarbolic acids |
1,631 |
0,013 |
0,033 |
±1,99 |
|
The
amount of phenilpropanoids |
1,537 |
0,014 |
0,036 |
±2,35 |