Chemistry and chemical technologies/6. Organic chemistry
Candidate of Chemistry A.K. Mamyrbekova
M. Auezov South Kazakhstan state university, Kazakhstan
Electrodeposition of copper
from water-dimethylsulphoxide
electrolytes
DMSO thoroughly dissolves inorganic
salts, particularly nitrates [1]. The solubility of copper(II) nitrate in DMSO
has not been studied. Water and DMSO are completely miscible. The excess
negative charge on the oxygen atom of the (ÑÍ3)2SO molecule
causes hydrogen bonding with both the hydrogen-containing molecules dissolved
in DMSO and the H atoms of the methyl groups in DMSO. Liquid DMSO is therefore
characterized by a definite level of structure [2] that is destroyed easily at
about 30°Ñ [3]. Since DMSO molecules are
cationotropic, they form quite strong complexes with copper(II) ions that have
coordination numbers from 2 to 4. There are data on the formation of stable [(ÑÍ3)2SO · NO3]– complexes in the presence of water [4]
when the nitrogen atom is linked directly with the sulfur atom, although the
possibility of such bonding was denied in [5]. The existence of Cu(NO3)2 · mDMSO
complexes where m is 2–4 was mentioned in [6].
The
physic-chemical properties of Cu(NO3)2.3H2O
in dimethylsulphoxide (DMSO), and also the cathodic deposition of
electroplatings copper in dependence from concentration (0.1-0.6 Ì), temperature (283-343 K) and current density (1-60 mÀ/sm2) have been investigated.
The new
electrolyte of copper on the basis of aprotonic polar solvent – dimethylsulphoxide
was elaborated and the optimum conditions permiting to obtain light, petty-crystal,
well cohesioned with basis deposits of high cleanliness were determined (Fig.1):
Cu(NO3)2.3H2O,
M – 0.1-0.4
jk,
mÀ/sm2 – 1-22
T, K - 283-298
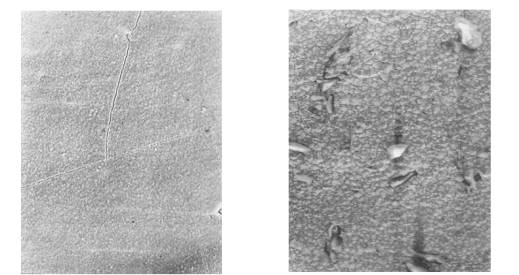
a b
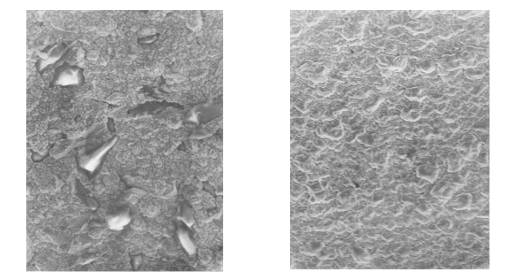
c d
Fig.1.
Morphology of deposits of the
copper received from 0,1 M of Cu(NO3)2.3H2O
in DMSO at jk = 5 mÀ/sm2 and different temperatures, K:
a – 288; b – 308; c – 318; d –
328 (increase in 2000 times).
The
dependence yield of copper from density of current about all investigated
concentrations expresses characteristic
curve analogous overturned parabola. From 0.1 M solutions curve was obtained
at jk=1-10 mÀ/sm2.
Increasing of concentration to 0.25 and 0.4 M stipulate for increasing of
maximum and widening of foundation parabols to 0.3-17 and 0.5-40 mÀ/sm2 respectively.
At the
high densities of current and temperatures the electroplatings soil with oxides, sulphides, that confirm dates roentgenographical analysis and
aggravation of appearance of plating. From 0.6 M solutions the yield current of
copper and quality obtained
electroplatings appreciablely decrease in all range of current densites.
The
electroreduction of the complex ions of copper(II) the composition [Cu(DMSO)4(H2O)2]2+ is proceeding in
two-stage on the adsorbate molecules of organic solvent electrode.
The
main kinetic parameters (coefficients of transfer a, heterogeneous constant of
velosity ks and effective energy of activation Aef) of
process electroreduction of the ions copper(II) in dimethylsulphoxide,
witnessing about reversible of process
were determined. The general velosity of cathodic process brakes of more slow
second stage, probably, having the mixed nature of control, as compared with
stage transfer of first electron was showed.
The
analysis of partial termodinamical parameters and physic-chemical properties of
investigated solutions and described results of experiment in electrodeposition
of galvanoplatings allows to make the following conclusion about dependence
of conditions electroreduction of
metall ions and formation of the cathodic deposit from condition the ions in solution: the delivery
electroactive ions to cathode, their discharge
and inclusion the atoms in cathodic deposit favourablely proceeding in certain
ranges of concentration, which coincides with maximum of mobility ions in
electrolyte. The conditions of maximum of mobility of the ions are determined
with conductometric method.
REFERENCES
1. A. V. Kolomiets
and N. D. Chkanikov, Chemical Encyclopedy (Sov. ntsiklopediya, Moscow,
1990), Vol. 2, p. 64 [in Russian].
2. V. I.
Skomorokhov and A. F. Dregalin, Zh. Fiz. Khim. 66, 2947 (1992).
3. D. Martin and H.
Hauthal, Dimethylsulfoxid (Academic_Verlag, Berlin, 1971).
4. H. L. Schlafer and
W. Schaffernicht, Angew. Chem. 72, 618 (1960).
5. F. Whitmore, Organic
Chemistry (van Nostrand, New York, 1987), p. 164.
6. Yu. N.
Kukushkin, Achievements of the Chemistry of Coordination Compounds (Naukova
Dumka, Kiev, 1975) [in Russian].