UDC 662.02: [531.72 + 544.33
Metaksa G.P., doctor of technical sciences
Buktukov N.S., doctor of technical sciences, professor
Moldabaeva G.J., candidate of technical sciences,
associate professor
The Republic of Kazakhstan, Almaty
Mining Institute after D.A. Kunaev
Modeling of System Bonds of
Fluid-Containing Macrosystems
The
paper shows that fluid-containing systems, including oil, can change their
physical properties under the impact of cross fields of various nature; and
this allows the implementation of the set mode of their parameters without the
application of energy intensive external impacts.
The concept on the natural
mode of deformation [1, 2, 3] of rocks establishes the periodicity of spatial
variability of their deformation behavior and residual stress in the blocks of
lithosphere of different structure. The existing charts of mathematic modeling
created with the purpose of long-term forecasting of variability of mechanical
and physical properties are quite correct in the limit of iteration step of the
model. At the same time they are not adequate for real systems participating
simultaneously in the complex transformation of incoming multidirectional
external impacts. In real system, consisting of structural elements of various
size, what matters is the main stress condition of the whole system needed to
maintain its balance. However it is known that it is easy to disturb the
balance with the cross impact on it with the efforts much lesser than the
potential of the main condition. Here is an appropriate illustration of tight
rope: the more is the tension the lesser effort is needed to destroy it at the
cross cutting loading.
Therefore in the experiment
conducted, the cross fields of various nature are used with the purpose of
control of the main compressive stress.
The table 1 shows the chart
of the factor analysis of the conducted experiments.
Table 1 – The chart of the factor analysis of the experiment on the modeling of system bonds of fluid-containing macrosystems
|
Kind of
fluid-containing system |
External impacts |
||||
|
Temperature |
Chemical |
Electrical |
Magnetic |
Resonant
|
|
|
Inorganic
minerals |
+ |
|
+ |
+ |
+ |
|
Organic
systems |
|
+ |
+ |
+ |
+ |
|
Water
solutions |
|
+ |
+ |
+ |
+ |
The purpose of the
experiment is to check the complex kinds of interaction of the system: “solid
substance – solution – gas”.
Source materials:
- Minerals of uniform composition;
- Soil of uniform
composition with humus content ~60% (black);
- Water solutions of various mineralization with
content of ions Í+, Ñl-, Na+;
- Films of mixed substances.
Methods of external impacts on the environment:
- Temperature;
- Resonant;
- Mechanical compression of solid phase;
- Complex.
Methods of control at the assessment of the outcomes:
- Measurements of electrical resistance;
- Measurements of ðÍ of solutions;
- Visual control;
- Scale measurements.
Table 2 – Values of electrical resistance for various forms of fluids
|
Kind of fluid |
electrical resistance,
kilohm |
|
Loose soil |
28 - 35 kilohm |
|
Soil solution of complete
saturation |
28 kilohm |
|
Pressed soil |
30 - 50 kilohm |
|
Saline soil (7%) |
14 kilohm |
|
Water solution 10% NaCl |
20 - 28 kilohm |
|
Water solution 7% NaCl |
14 kilohm |
|
Water solution 5% NaCl |
12 kilohm |
|
Water solution 2% NaCl |
12 - 16 kilohm |
|
Water solution of stearate
Na - 1% |
50 - 60 kilohm |
|
Water solution of trisodium
phosphate |
55 - 60 kilohm |
|
Water solution ÍCl -10% |
10 - 11 kilohm |
|
Water solution ÍCl -5% |
16 kilohm |
|
Water solution ÍCl -2% |
14 kilohm |
|
Water solution ÍCl -0,5% |
12 kilohm |
|
Water solution ÍCl -0,01% |
18 kilohm |
|
Water solution ÍCl -0,001% |
20 - 22 kilohm |
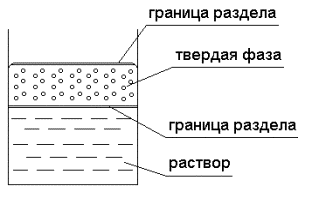
The picture 1 shows the functional chart of experiment
conduction with fluid-containing minerals, on which the main kind of impact is
compressive force Ð, and for
controlling (cross) the weak impacts of various nature are used (chemical,
electrical, magnetic, temperature).
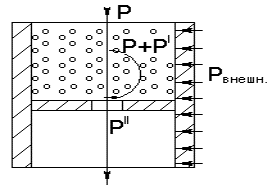
Ð- compressive force,
Ð+Ð'- the same with due account to the
shearing component,
Ðâíåøí.- external impacts (chemical, electrical,
electromagnetic) mechanical
Ãðàíèöà ðàçäåëà – interface
Òâåðäàÿ ôàçà – solid phase
Ðàñòâîð -solution
Picture 1 – The chart of
complex impact on fluid-containing solid phase
After the conduction of
tests according to the shown chart, the pressed block of solid phase is
resulted in the outcome, the electric properties of which are measured in
mutually perpendicular directions and at the interfaces of phases on the block.
To receive the uniformity of content and humidity, the soil was dried out in
kiln and screened, filling it before the experiment with the same dose of
solutions of various mineralization. The temperature range of the studies did not
exceed 700Ñ (maximum possible
temperature in real conditions), frequency range corresponds to the spectrum of
the used generator GZ-120.
Below are given the values
of electrical resistance ![]() of the solid phase depending on the change of temperature of experiment,
concentration of water solutions, acid-alkali parameters and solid phase.
of the solid phase depending on the change of temperature of experiment,
concentration of water solutions, acid-alkali parameters and solid phase.
On the picture 2 it is
possible to identify two types of curves ![]() depending on the kind of external impact: below three exponential curves
can be seen (such are drawn at the solution of differentials of the type
depending on the kind of external impact: below three exponential curves
can be seen (such are drawn at the solution of differentials of the type ![]() ), which arise at the impact of one kind of impact gradients. At the
complex impact (2 - 3 kinds of impacts with frequencies of neighboring levels –
upper curves) spasmodic (more than 10 times) increase of electrical resistance
arises due to the change of chemical content of the solid phase. This is an
indicator of the fact that this system is in autowave mode of interaction, and
it significantly differs from usual behavior of the system at the static chart
(lower curves).
), which arise at the impact of one kind of impact gradients. At the
complex impact (2 - 3 kinds of impacts with frequencies of neighboring levels –
upper curves) spasmodic (more than 10 times) increase of electrical resistance
arises due to the change of chemical content of the solid phase. This is an
indicator of the fact that this system is in autowave mode of interaction, and
it significantly differs from usual behavior of the system at the static chart
(lower curves).
![]()
![]()
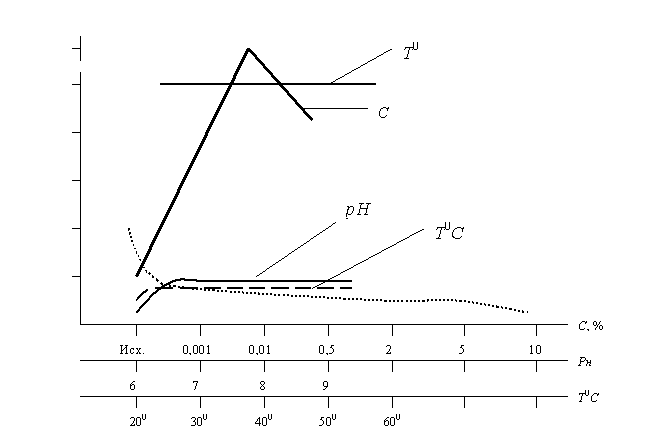

Picture 2. Change of electrical resistance depending on the
concentration (Ñ,%), temperature (Ò0Ñ) and ðÍ of solutions
Besides, some particular regularities of interactions
with the neighboring levels have been revealed. So, mechanical variations
(range of 300 - 400 Hz) accelerate chemical reactions only at the interface of
phases.
Addition of sodium chloride to the solution (change of
quantity of degrees of freedom of the system), organic molecular compounds
(sodium stearate, trisodium phosphate) result in appearance of anisotropy of
properties in the solid phase by means of creation of unbalanced conditions.
By changing the direction and the form of impacts at
the interface of phases it is possible to control (intensify or neutralize) the
properties both of the interface itself and interphase area.
Unbalanced conditions in solutions can be modeled by
creating either counter flow of one quality or two different, angularly
directed flows. In the case under consideration, the temperature gradient,
constant magnetic field (200 oersteds) and concentration gradient have been
used.
Thus it was revealed that the filtration coefficients
do not depend on the change of magnetic and temperature parameters of
environment (in the limits of conditions of summer season) and are quite
sensible to the change of concentration of solutions and capillary diameter
(see table 3), and the speed of evaporation, in opposite, depends strongly on
them.
Table 3 – The change of values of filtration speeds and evaporation for
capillaries of various size
|
speed of filtration (mm/sec) for cappilaries (mm) |
|||||
|
kind of fluid |
1,0 |
0,5 |
0,1 |
<0,1 |
speed of evaporation mm/hr |
|
solution 10% nacl |
0,4 |
1,0 |
0,6 |
0,02 |
0,156 |
|
solution 5% nacl |
2,0 |
1,0 |
0,6 |
0,2 |
0,156 |
|
solution 2% nacl |
1,6 |
1,0 |
0,6 |
0,4 |
0,09 |
|
10% nacl+0,5% na stearate |
0,6 |
0,2 |
1,0 |
0,01 |
0,156 |
|
5% nacl+0,5% na stearate |
1,0 |
1,2 |
0,6 |
0,01 |
0,156 |
|
2% nacl+0,5% na stearate |
1,0 |
1,2 |
0,6 |
0,01 |
0,19 |
|
10% nacl in magnetic field |
0,6 |
0,4 |
0,6 |
- |
0,08 |
|
5% nacl in
magnetic field |
1,0 |
1,2 |
1,0 |
- |
0,08 |
|
2% nacl in magnetic field |
1,0 |
1,2 |
1,0 |
- |
0,09 |
For coefficients of macrodispersion, the strongest
dependence on the size of contacting phases, on the direction of magnetic field
and on the quality of solution (acid-alkali parameter) has been found.
Table 4 – The values of coefficients of macrodispersion depending on the
concentration and size of contacting layers
|
coefficient of
macrodispersion, m2/day |
|||
|
concentration of salt solution NaCl |
1 component (1 degree of freedom), m2/day |
2 components (different sings of gradient |
1 component, relation of strata sizes 1:3 |
|
10% |
6,27*10-2 |
31,2*10-2 |
18,9*10-2 |
|
5% |
6,27*10-2 |
31,2*10-2 |
18,9*10-2 |
|
2% |
18,9*10-2 |
18,9*10-2 |
37,8*10-2 |
The table shows that according to the order of values,
the measured coefficients correspond to the experimental values given by
J.Freed for the calculation of salt transportation at the use of dispersion
chart (1*10-2m2/day). This value by three ranks (~ 1*10-5
m2/day) differs from the most frequently applied value of the
dispersion coefficient. Besides, the table shows that these coefficients in the
limits of one rank can depend on the concentration of both lower and upper
layers of the model. The hyphen depends on the position of more saturated
(concentrated) layer between which oscillation process often arises. As a
result some optimal concentration level is received around which the system
maintains the balance. In the case under consideration this concentration is
near to 5% NaCl. Shifting the balance of this system by introducing another
components (poling molecular compound), it is possible to change the
oscillation mode toward any direction in the limits of its level of
consideration. So, in the solution with 5% NaCl it is possible to observe the
appearance of “salt fingers” with different spatial orientation depending on
the kind of external impact (electromagnetic, thermal etc.)
The coefficients of macrodispersion in these cases
have the following values:
14,2*10-2 m2 /day – for 10% NaCl
solution
23,2*10-2 m2 /day – for 5% NaCl
solution
8,4*10-2 m2 /day – for 2% NaCl
solution
Scale factor (relation of height and diameter of the
modeled layers 1:2 and 1:3) to much extent affects the values of dispersion by
changing its values by 2-3 times in comparison to the input ones; at that for
small concentrations the scale effect is the most essential.
The change of the structure of the surface layer or
interface between the phases also significantly affects the speed of
evaporation and macrodispersion, where the difference can exceed the input
values of solutions by 1-2 times.
The
analysis of the results of multifactor experiment allows making the following
conclusions:
1.
Fluid-containing systems, including oil, can change their physical
properties under the impact of the cross fields of various nature; and this allows the implementation of the set mode of their parameters
without the application of energy intensive external impacts. For instance, it
is possible to change oil viscosity without heating.
2.
At the cross impacts it is necessary to consider the size and time
relations in the system “impact-response” as well as the structural
particularities of interfaces.
Literature
1. Chabdarova Yu.I.,
Jujgov Yu.V., Bukin A.N. Mining pressure in anticline structures of Djezkazgan,
Alma-Ata, Nauka, 1980, 195 p.
2. Aitmatov I.T.
Geomechanical conditions in zones of sources of bounces and technogeneous
earthquakes. Bishkek, 2002. p. 3-84.
3. Rasskazov I.Yu.
Control and management of mining pressure at mines of Far East region. Ì., Gornaya kniga, 2008, 329 P.