Pisanenko D.A., Grib
О.К.
National Technical University of Ukraine
«Kiev Polytechnical Institute»
Pesticidal properties of quaternary
ammonium salts based on chloromethylated diarylmetanes
dpisanenko@mail.ru
It is known
that quaternary ammonium salts are the basis of a number of industrial
pesticides used in agriculture [1]. However, the observed increase in
resistance of microorganisms and fungi to these pesticides causes the synthesis
and study of the pesticide properties of new salts of this series [2,3].
Practical interest can provide quaternary ammonium salts derived from available
commercial reagents, which include diarylmetanes used as liquid dielectrics,
heat transfer fluids and plasticizers [4,5]. In this regard, the purpose of
this work was to study the synthesis and properties of the pesticide quaternary
ammonium salt-based products such diarylmetanes chloromethylation.
As
diarymetanes used mono-, di-and tribenzyltoluens isolated by fractionation of a
commercial mixture and the products of benzylation benzyltoluens xylene,
ethylbenzene, o-chlorotoluene and phenetoule, as determined using the GLC on
the device "Biohrom-3700" (5% OV-17 on Chromosorb W, carrier gas -
helium). Chloromethylation with control groups of CH2Cl-NMR Spectrometer (TESLA
BS-487, internal standard HMDS) and quaternization was performed as previously
described [6]. In this scheme, we have stated below were synthesized quaternary
ammonium salts (1-10):
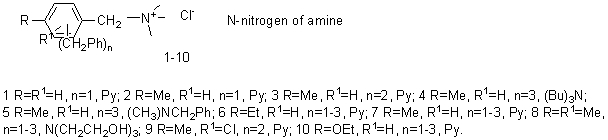
Pesticidal properties of the synthesized compounds
(1-10) were studied by standard
methods adopted for carrying out screening studies [7]. The experimental results are presented in Tables 1-2.
Table 1
Insecticidal and aphicidal properties of
the compounds (1-10)
|
Com-pound |
The death of insects
and mites,% |
|||
|
Houseflies |
Beetles of
rice weevil |
spider mite |
Black beet
aphids |
|
|
1 |
0 |
20 |
62 |
57 |
|
2 |
15 |
20 |
65 |
9 |
|
3 |
55 |
72 |
40 |
47 |
|
4 |
59 |
52 |
13 |
8 |
|
5 |
38 |
7 |
60 |
8 |
|
6 |
44 |
97 |
42 |
0 |
|
7 |
51 |
2 |
25 |
13 |
|
8 |
41 |
42 |
50 |
0 |
|
9 |
84 |
42 |
0 |
12 |
|
10 |
77 |
22 |
18 |
7 |
As can be seen from Table 1, compounds
(1-10) exhibit a moderate activity against insects and spider mites, little effect on the black beet aphids.
Only compound (3) and (6) show significant
activity against rice weevil beetles (72
deaths and 97% respectively), compound (9) and (10) is
sufficiently active against houseflies
(84 deaths and
77% respectively).
Compounds (1-10) have a weakly pronounced
fungicidal and bactericidal properties (Table 2). The maximum effect on smut (72%
inhibition of development) has only the compound
(10).
Table 2
Fungicidal and bactericidal activity
of the compounds (1-10)
|
Compound |
The percentage of
inhibition of growth and development of colonies of bacteria and fungi,% |
|||||||
|
Xanth.malv. |
Fusar. monilif. |
Vertici. dachli. |
Ventur. inaequ. |
Botryt. cinerea |
smut |
phytophthora |
mildew |
|
|
1 |
0 |
14 |
0 |
0 |
0 |
0 |
0 |
0 |
|
2 |
14 |
22 |
25 |
0 |
0 |
0 |
0 |
53 |
|
3 |
0 |
32 |
11 |
0 |
0 |
0 |
48 |
51 |
|
4 |
14 |
15 |
6 |
7 |
13 |
25 |
0 |
29 |
|
5 |
0 |
15 |
13 |
7 |
0 |
56 |
0 |
51 |
|
6 |
7 |
11 |
0 |
7 |
0 |
63 |
0 |
56 |
|
7 |
25 |
31 |
17 |
23 |
17 |
0 |
33 |
0 |
|
8 |
12 |
51 |
11 |
23 |
8 |
41 |
33 |
0 |
|
9 |
25 |
44 |
0 |
11 |
33 |
0 |
0 |
0 |
|
10 |
11 |
0 |
7 |
0 |
10 |
72 |
0 |
0 |
Herbicidal activity was studied in 11 test sites
in the greenhouse under optimal lighting conditions, humidity and temperature.
Compounds (1-10) at a dose corresponding to 5 kg /
ha, applied in two ways: by
spraying the soil after sowing
of seeds and growing plants by spraying. Showed high
herbicidal activity of the compound (6,9-10),
they caused a complete loss of radish and
53-62% reduction in weight of buckwheat.
From the results it follows that among the synthesized quaternary ammonium salts (1-10) compounds are present, pesticide properties are of interest to develop new chemicals and to study the
relation between structure and biological
activity.
Literature:
1. Справочник по пестицидам. Под ред. А.В.Павлова,
Киев:Урожай,1986. 432 с.
2. Sharma ML., Bangar
J., Singh R. Pest.Res. Journal. 2008. V.20.N2. P.178-182.
3. Ahmed S.M., Ismail
D.A. J.Surfc .Detergents. 2008. V.11.N3. P.231-235,
4.
Матвеев И.Г., Гальперин
Н.И., Вильшау К.В. и др.// Журн.прикл.химии. 1958. Т.31. №6. С.869-874.
5.
А.с. 1498746 СССР. МКИ
СО7с15/18. Способ получения моно- и дибензилтолуолов.
6.
Писаненко Д.А.,
Погребова И.С., Авилов В.О. и др.// Журн.прикл.химии. 2005. Т.78. №9.
С.1475-1478.
7.
Писаненко Д.А., Гриб
О.К. Сб.докл.Междунар.научно-практ.конф. «Achiement of High School-2011». София, 2011. Т.28. С.36-39.