Models of biocatalysts on the basis of complexes of
pseudo-interpenetrating polymer nets with transitional metals ions
Bektenova G.À.*, Chinibaeva N.S., Bekturov
E.À., Zhanbekov H.N., Orazbaeva M.A.
A.B. Bekturov Institute of Chemical
Sciences, KazNPU named after Abai,
Almaty, Republic of Kazakhstan, Sh.
Walikhanov Str., 106
E-mail: chinibayeva@mail.ru
Abstract: The
work presents the study of complex-formation of interpenetrating nets with
transient metals. Catalase activity of the obtained systems has been studied in
the reaction of decomposition of hydrogen peroxide.
Key words: biomimetics,
catalse activity, polymer-metallic complexes, biocatalysts, interpenetrating
nets.
Introduction
In the recent years an ever greater attention is paid to the problems of molecular engineering and biomimetics. Polymer catalysts, including in their composition complexes of transient metals, are of special interest. These catalysts combine all advantages of heterogenic catalysts, such as a possibility of separation from the reaction medium, repeated use, and a high stability with high actiity and selectivity of homogeneous catalysts. Earlier we have studied complexes of hydrogel of polyacrylic acid and polyethyleneimine with transient metals [1,2]. It has been shown that the complexes of hydrogel of polyethyleneimine with transient metals display a higher activity in comparison with the complexes on the basis of hydrogel of polyacrylic acid and salts of transient metals. The most interesting are interpenetrating polymer nets (IPN), in which the functional groups are available for the coordination with ions of metals and the consequent interaction of the coordination centers with the substrate. Interpenetrating nets can be characterized as a carcass, consisting of the combination of two reticulated polymers. One can single out two signs, in which IPN differ from the simple polymer mixtures, block polymers and engrafted copolymers: 1) IPN swell, without dissolution, in solvents, and 2) their rheological properties are limited [3].
Experimental
Iron (III) chloride, copper (II)
chloride, cobalt (II) chloride, nickel (II) chloride corresponded to the
qualification «chemically pure». A swelling coefiicient of IPN was determined
by the method of gravimetry.
ðÍ of the solutions was measured on ðÍ-meter ÎÐ-264/1 («Reanal», Hungary) with the accuracy up to 0,03 unit of ðÍ. Catalase activity of the complex was determined by the modified methods [4] and it was expressed in the units of enzymic activity per 1g of dry gel.
Results and Discussions
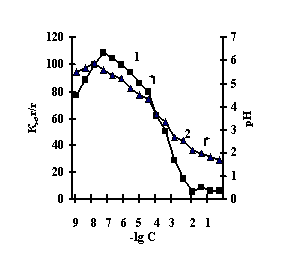
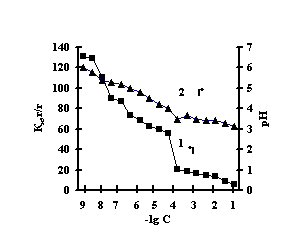
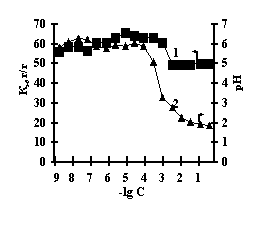
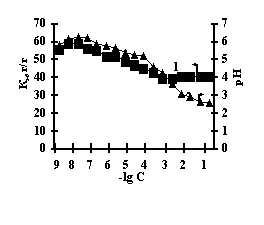
With the purpose of obtaining of biomimetic systems we studied complex-formation of IPN on the basis of agar-agar and polyacrylic acid (PAA), agar-agar and polyethyleneimine (PEI) with the chlorides of copper (II), iron (III), cobalt (II), nickel (II). The reaction of complex-formation of IPN with transient metals (Ìe) is accompanied by the compression of the net and a decrease in pH of the solution with an increase in the concentration of salt. For the system of FeCl3-Àg-Àg-PAA 'Figure 1a, curve 1'a moderate decrease of the coefficient of swelling is observed with the salt concentration of 1õ10-5Ì, and for the remaining systems the coefficient of swelling decreases in the range of 1õ10-2Ì – 1õ10-1Ì 'Figure 1 b,c,g'.
|
à |
b |
|
c |
g |
Fig. 1 Dependence of a swelling coefficient (1) and ðÍ of the IPN (2) on the basis of agar-agar-PAC on the
salt concentration: (à) FeCl3,
(b) CuCl2, (c) CoCl2, (d) NiCl2
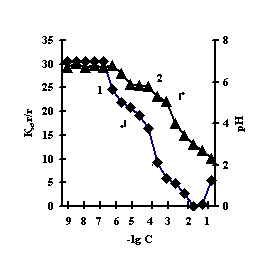
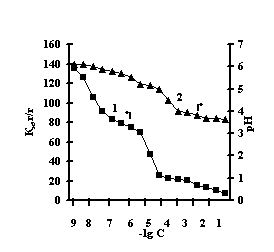
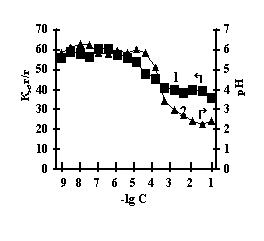
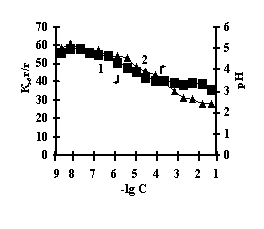
This is connected with electrostatic interactions between the ions of metal and carboxyl groups of PAA, as a result of which hydrogen ions of PAA carboxyl groups are isolated into the solution, and Àg-Àg fulfills the role of a carcass in the polymer net. An insignificant decrease of the coefficient of swelling of Àg-Àg-PEI is characteristic for the system Men+-Àg-Àg-PEI 'Figure 2 a,b,c,g, curve 1'. An insignificant decrease of the coefficient of swelling of Àg-Àg-PEI can be stipulated by the rigidity of the structure of the natural polymer gel, inside which there is a linear ramified synthetic macromolecule of PEI in the interweaning of polysaccharide.
|
à |
b |
|
c |
g |
Fig. 2 Dependence of a swelling coefficient (1) and ðÍ of the IPN solution (2) on the basis of agar-agar-PEI
on the salt concentration: (à) FeCl3,
(b) CuCl2, (c) CoCl2, (d) NiCl2
Catalase activity of the given complexes
was determined by the permangantometric method [4]. The reaction of
decomposition of hydrogen peroxide was carried out in 0,1Ì of phosphate buffer solution
at ðÍ=7,0. It
was shown that the complexes of IPN on the basis of agar-agar and
polyetyhyleneimine with the salts of transient metals display a higher activity
than nthe complexes of IPN on the basis of agar-agar and polyacrylic acid with
the salts of ransient metals. The reason of the high activity of complexes of Ìå-Àg-Àg-PEI is stipulated by the presence of a cation polymer, in
the chain of which thre are iminogroups, entering a donor-acceptor bond with
the ions of metals. The same activity was observed when studying the activity
of the previously obtained complexes on the basis of hydrogels of polyacrylic
acid and polyethyleneimine with the salts of transient metals [5]. An ion of
metal, which possesses d-orbitals, serves as an acceptor, a nitrogen atom,
offering a pair of electrons for the formation of a net serves as a donor.
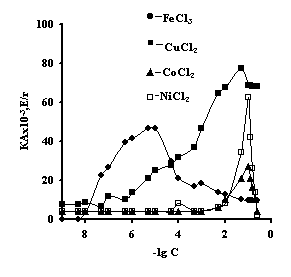
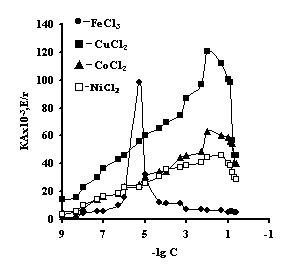
It is noteworhy that the catalase activity of the complexes of iron with IPN is maximal with small concentrations of salt, with th concentration of salt of 1õ10-5Ì 'Figure 3'. For the complexes of copper, cobalt, nickel with IPN the catalase activity is maximal in the range of salt concentration of 1,0õ10-2 – 1,0õ10-1Ì 'Figure 3' . It is probably connected with the nature of ion of metal, which is confirmed by the literature data [6]. By the displayed activity they can be arranged in the row: NiCl2-Àg-Àg-PAA < ÑîÑl2-Àg-Àg-PAA < FeCl3-Ag-Àg-PAA < CuCl2-Àg-Àg-PAA; NiCl2-Àg-Àg-PEI < ÑîÑl2-Àg-Àg-PEI < FeCl3-Ag-Àg-PEI < CuCl2-Àg-Àg-PEI.
|
à |
b |
Fig. 3 catalase
activity of IPN on the basis of agar-agar and à) PAC
with the ions of transient metals; and b) PEI with the ions of transient metals; t=3 minutes, ðÍ = 7,0, T=25ºÑ, C (H2O2) = 0,12Ì.
The study of the depndence of the activity of complexes upon ðÍ of the medium has shown that the activity of complexes is maximal at ðÍ=7,0, which is characteristic for a native enzyme. The study of ðÍ-stability of complexes upon their incubation for 4 hours showed that the activity of Àg-Àg-PAA-Ìå and Àg-Àg-PEI-Ìå is maximal in the range of 5,0-8,0, where they are most stable and more than 80% of the initial activity is preserved for the complexes of complexes of FeCl3-Àg-Àg-PAA and FeCl3-Àg-Àg-PEI and more than 60% - for the complex of Àg-Àg-PAA-Ìå and Àg-Àg-PEI-Ìå. By ðÍ-stability the same row is observed as for that for the displayed activity. Such a picture is connected with the structure of polymer-metallic complexes, the character of the in teraction of the functional groups with an ion of metal and the interaction of the given systems with hydrogen peroxide. The systems on the basis of pseudo-interpenetrating nets with ions of iron are most interesting as models of biocatalysts, because even with a small concentration of salt the complexes with IPN are formed, which display the maximal activity. It is also connected with the structure of catalase, the peculiar feature of which is the presence of «natural mark» in a prostetic group – gemic iron, in which an ion of iron is in the three-valent state. Though the activity of the complexes of IPN with ions of copper is much higher than that of the IPN complexes with ions of iron. However, the IPN complexes with ions of iron are more stable than the IPN complexes with ions of copper. Hig catalytic activity and specificity of natural catalysts – enzymes, as a rule, is stipulated by the combined participation in the catalysis of the functional groups of different nature, located in definite cells of the polymeric matrix. It is probable that upon the diffusion of ions of metals in the polymer net coordination centers are formed, including either different or similar functional groups. An optimal location of functional groups in relation to each other, ensuring the structural correspondence of a catalyst and a substarate.
Conclusion
Thus,
from the obtained experimental data one can conclude that complex formation
between ions of metal and IPN has a complex character, and proceeds by
different mechanisms. Insidentally, many factors produce a strong effect on the
depth of binding and properties of the formed complexes. The obtained
biomimetic systems on the basis of the above-mentioned complexes, with the
purpose of creation and study of polymeric catalysts, modeling the enzyme
catalase, display a high catalase activity
in the reaction of decomposition of hydrogen peroxide.
References
1.
Bektenova
G.A., Chinibaeva N.S., Bekturov E.A.: Biocatalytic
Activity of Polyethylene
Imine Complexes with
Transition Metal Ions , Proc. Of MRS
Fall Meeting 2005 (MRS Fall 2005), Boston, USA, Full Paper 0897 – J03 – 11. – 6p. (2005).
2.
Bektenova G.À., Chinibaeva N.S., Bekturov Å.À.: The complexes of ferric salts
with polymer hydrogels and investigation of its biocatalytical activity. Izvestiya
of STS «Kakhak», 3, 14-18 (2006).
3.
Sperling
L.: Interpenetrating polymer nets and analogous
materials. Ì.: MIR, (1984).
4.
Bektenova G.A.: Interaction of Catalase with
Cationic Hydrogels: Infiuence of pH, Kinetics of Process and Isotherms of
Adsorption, J. Izvestiya. MES RK, 3, 82-91 (2001).
- Chinibaeva N.S., Bektenova G.À., Beturov Å.À.: Study of biocatalytic activity of the complexes of hydrogel of
polyethyleneimide with the ions of transient metals. Proceedings of the International
Scientific-Practical Conference «Academician Å.À.Buketov-scientist, pedagogue, thinker».-
Karaganda, Kazakhstan, 487-488 (2005).
- Nikolaev
L. À.: Biocatalysts
and their models. Ì.,
(1968).