UDC 669.053.2
N. Komkov (EKSTU, Ust-Kamenogorsk),
V. Luganov (KazNTU, Almaty)
PECULIARITIES OF POLYMETALIC SULPHIDE
CONCENTRATE SOLID-STATE OXIDATION
Sulphide
ores and concentrates are usually the charging feed for most metals production.
Processing of the materials in order to get metals is connected with the
process of sulphide oxidation. In metallurgical processes sulphide oxidation is
realized in solid and liquid states.
In many cases sulphide materials roasting is the first stage of processing when solid sulphides are oxidized. In other cases, e.g. while smelting, sulphide oxidization is carried in both liquid and solid states. And finally there are some processes in which oxidization is carried entirely in liquid state. Converter blowing the matte in order to get crude copper and autoclave leaching are examples of them.
Nowadays low-grade
concentrates are being processed. In applied sciences the task of finding these
concentrates’ processing modes arises. That proves the great importance of studying
the principles of sulphide oxidization processes in metallurgy.
The choice of thermal treatment method is determined by the character of
the antecedent and subsequent processes, types of energy sources in the industry
being designed and by many other factors including physical and chemical
characteristics of the processed material. The tasks of material processing technology
development include choosing a rational method and the optimum mode that will
ensure acquiring the target product quality based on the characteristics of the
processed material and on the certain industry conditions. At present time roasting
in fluid-bed furnaces is a conventional method of pyrometallurgical treatment
of sulphide materials.
The optimum mode is understood as the one which provides the best quality
of the product with the minimum reagents and energy consumption. Temperature
mode and technological parameters depend on the material characteristics. The maximum
fluid-bed temperature permissible within the limits determined by the material
characteristics should be provided in order to intensify the process. Off-gas temperature
is specified by the kinetic peculiarities of sulphate formation.
The roasting process should be intensified
with elimination of impermissible strains in the material and overheat that can
cause formation of liquid phases.
As regards the
intensification, the right choice of temperature and hydro-dynamical mode seems
to be very important. The choice of temperature mode is predetermined by the chemical
composition of the charging feed and by the processes occurring in it while
being heated and roasted. Thus, before
choosing the roasting mode (temperature, gas velocity, concentration and etc.) it’s
necessary to study beforehand the material and end product response in a wide temperature
range as in the certain change intervals of moisture (and temperature) sharp
charging feed phase fluctuations caused by the temperature and the content
(fluxation, melting and decomposition) can be observed. Being aware of these process
peculiarities for every temperature interval it’s possible to use individual
most intensive roasting mode as well as to evaluate the possible deviation of
mode parameters [2]. Concentrate ¹1 unlike Concentrate ¹2 contains the minimum amount
of impurities and doesn’t cause significant difficulties while being roasted
that is why it was determined as the check standard.
Concentrates description
Table 1- Chemical composition of zinc sulphide
concentrates
|
|
Concentrate
¹ |
Elements |
||||||
|
Potential, eV |
Zn |
Fe |
Cu |
Pb |
SiO2 |
S |
Sum of impurities |
|
|
0.069 |
1 |
53.3 |
6.2 |
0.28 |
0.61 |
2.6 |
32.6 |
9.69 |
|
0.119 |
2 |
45.8 |
8.2 |
0.9 |
2.12 |
4.8 |
30.7 |
16.02 |
|
0.327 |
3 |
43.2 |
7.1 |
4.1 |
2.9 |
4.4 |
29.6 |
18.5 |
|
0.539 |
4 |
37.1 |
5.6 |
0.10 |
4.7 |
8.8 |
24.7 |
19.2 |
|
0.687 |
5 |
41.8 |
7.8 |
2.7 |
4.2 |
7.4 |
29.7 |
22.1 |
|
0.888 |
6 |
44.7 |
7.2 |
2.9 |
4.9 |
8.3 |
24.1 |
23.3 |
|
1.0195 |
7 |
40.8 |
10.5 |
3.2 |
4.7 |
6.5 |
30.6 |
24.9 |
Concentrate ¹1
Concentrate ¹1 possesses the following
mineral composition,%: 79.44 ZnS, 9.99 FeS2, 0.70 PbS, 0.81 CuFeS2,
2.60 SiO2, less than 0.5 of fahlore. All the minerals of the
concentrate are represented as angular flakes. 40–50% of ZnS sphalerite is in free
form. The rest of sphalerite has emulsion impregnation of CuFeS2 copper pyrite 1 – 5 microns in size as
well as copper pyrite, FeS2 pyrite, PbS galenite inclusions or marginals 10 – 20 microns in size.
Concentrate ¹2
Concentrate ¹2 possesses the following
mineral composition,%: 68.26 ZnS, 10.25 FeS2, 4.16 FeS, 2.45 PbS, 2.6
CuFeS2, 4.8 SiO2, less than 0.5 of fahlore.
Significant diversity in the
concentrate structure is not observed. Singular pyrite and copper pyrite flakes are noticed.
Concentrate
¹3
Concentrate ¹3 possesses the following
mineral composition,%: 64.4 ZnS, 6.77 FeS2, 0.53 FeS, 3.4 PbS, 11.80
CuFeS2, 4.4 SiO2, less than 0.5 of fahlore.
It does not differ from Concentrate ¹1
in structure. ZnS sphalerite is mostly in free form and rarely in crystal-jams
with CuFeS2 copper pyrite.
Concentrate
¹4
Concentrate ¹4 possesses the following
mineral composition,%: 55.29 ZnS, 9.39 FeS2, 1.802 FeS, 5.43 PbS, 0.288
CuFeS2, 8.8 SiO2, less than 0.5 of fahlore.
Concentrate
¹5
Concentrate ¹5 possesses the following
mineral composition,%: 62.3 ZnS, 10.13 FeS2, 1.13 FeS, 4.85 PbS, 7.79
CuFeS2, 7.4 SiO2, less than 0.5 Cu5FeS4.
It differs from Concentrate ¹1 in the presence of epigenetic sulphides and Cu5FeS4
erubescite.
Concentrate
¹6
Concentrate ¹6 possesses the following
mineral composition,%: 63.17 ZnS, 7.34 FeS2, 5.66 PbS, 7.63 CuFeS2,
8.3 SiO2. It does not differ from Concentrate ¹1 in structure. Most
part of ZnS sphalerite is in free form. Inclusions are represented by emulsion
impregnation of CuFeS2 copper pyrite. Sulphur deficit is 6.8 %, in sulphurite – 3.43 %, in copper pyrite – 0.72
%, in pyrite – 2.65 %.
Concentrate
¹7
Concentrate ¹7 possesses the following mineral composition,%: 60.8 ZnS,
8.3 FeS2, 6.03 FeS, 5.43 PbS, 9.24 CuFeS2, 6.5 SiO2,
less than 0.5 of fahlore. It differs from Concentrate ¹1 by the presence of epigenetic
cupric sulphides, 7Cu5FeS4 erubescite, Cu2S
chalcosine and covelline CuS.
The Results of Low Grade Zinc
Sulphide Concentrates Roasting in Laboratory Furnace
The research of low grade zinc sulphide
concentrates roasting was conducted in a lab pipe furnace of SUOL – 0,4.4/12 type with the temperature of 1173 – 1273 Ê in blast air. The blast air velocity
was kept at 2 dm3 per minute by ejector regulation
of exhaustion and thrust power in gas duct system and by controlling blast rate
with a flow gauge. The furnace temperature was automatically kept and controlled
with platinum-rhodium thermal converter and with a secondary instrument that
was a millivoltmeter. Zinc sulphide concentrate of 10 gram weight quantity in a
porcelain boat was placed into quartz tube installed in the furnace. The research
duration was within 1600 – 7200 second duration.
Table 2 - Roasted Product of
Zinc Concentrate ¹1
|
Time,
s |
Content,% |
|||||||||||
|
Zno |
Znê |
Znâ |
Fe |
Cu |
Pb |
SiO2 |
So |
SSO4 |
Ss |
Zn Solub., % |
Desulph.,% |
|
|
1800 |
58.1 |
18.9 |
0.21 |
6.76 |
0.305 |
0.66 |
2.83 |
20.5 |
0.45 |
20 |
32.53 |
37.12 |
|
3600 |
60.5 |
34.8 |
0.19 |
7.04 |
0.32 |
0.69 |
2.95 |
12.5 |
0.38 |
12.12 |
57.52 |
61.66 |
|
5400 |
62.5 |
48.5 |
0.13 |
7.27 |
0.33 |
0.72 |
3.05 |
5.3 |
0.38 |
4.92 |
77.6 |
83.74 |
|
7200 |
63.6 |
55.4 |
0.12 |
7.398 |
0.334 |
0.73 |
3.102 |
2 |
0.35 |
1.65 |
87.11 |
93.86 |
Table 3 - Roasted Product of Zinc Concentrate ¹2
|
Time,
s |
Content,% |
|||||||||||
|
Zno |
Znê |
Znâ |
Fe |
Cu |
Pb |
SiO2 |
So |
SSO4 |
Ss |
Zn Solub., % |
Desulph.,% |
|
|
1800 |
51.4 |
19.3 |
0.42 |
9.2 |
1.01 |
2.38 |
5.39 |
17.9 |
0.71 |
17.9 |
37.55 |
41.69 |
|
3600 |
53.2 |
30.2 |
0.56 |
9.52 |
1.04 |
2.46 |
5.58 |
11.5 |
0.8 |
10.7 |
56.77 |
62.54 |
|
5400 |
53.9 |
38 |
0.41 |
9.65 |
1.06 |
2.49 |
5.65 |
7.2 |
0.73 |
6.47 |
70.5 |
76.55 |
|
7200 |
55.3 |
34.1 |
0.16 |
9.9 |
1.09 |
2.56 |
5.8 |
4.2 |
0.68 |
3.72 |
61.66 |
86.32 |
Table 4 - Roasted Product of Zinc Concentrate ¹3
|
Time,
s |
Content,% |
Zn Solub., % |
Desulph., % |
|||||||||
|
Zno |
Znê |
Znâ |
Fe |
Cu |
Pb |
SiO2 |
So |
SSO4 |
Ss |
|||
|
1800 |
46.9 |
13.1 |
0.39 |
7.71 |
4.45 |
3.15 |
4.78 |
20.3 |
0.44 |
19.86 |
27.93 |
31.42 |
|
3600 |
48.3 |
17.8 |
0.17 |
7.94 |
4.58 |
3.24 |
4.92 |
16 |
0.35 |
15.6 |
36.85 |
45.94 |
|
5400 |
49.9 |
25.8 |
0.11 |
8.2 |
4.74 |
3.35 |
5.08 |
10.6 |
0.4 |
10.2 |
51.7 |
64.19 |
|
7200 |
50.3 |
34.2 |
0.07 |
8.27 |
4.77 |
3.38 |
5.12 |
4 |
0.53 |
3.47 |
67.99 |
86.49 |
Table 5 - Roasted Product of
Zinc Concentrate ¹4
|
Time, s |
Content,% |
Zn Solub., % |
Desulph.,% |
|||||||||
|
Zno |
Znê |
Znâ |
Fe |
Cu |
Pb |
SiO2 |
So |
SSO4 |
Ss |
|||
|
1800 |
48.4 |
23.8 |
0.26 |
7.31 |
0.13 |
6.13 |
11.48 |
12.2 |
0.82 |
11.38 |
49.17 |
50.61 |
|
3600 |
50.7 |
32 |
0.3 |
7.65 |
0.14 |
6.42 |
12.03 |
8.1 |
0.96 |
7.14 |
63.12 |
67.21 |
|
5400 |
51.6 |
34.3 |
0.42 |
7.79 |
0.14 |
6.54 |
12.24 |
7 |
0.95 |
6.05 |
66.47 |
71.66 |
|
7200 |
51.9 |
44 |
0.17 |
7.83 |
0.14 |
6.58 |
12.31 |
1.9 |
1 |
0.9 |
84.78 |
92.31 |
Table 6 - Roasted
Product of Zinc Concentrate ¹5
|
Time, s |
Content, % |
Zn Solub., % |
Desulph.,% |
|||||||||
|
Zno |
Znê |
Znâ |
Fe |
Cu |
Pb |
SiO2 |
So |
SSO4 |
Ss |
|||
|
1800 |
42.8 |
13.7 |
0.45 |
7.99 |
2.76 |
4.3 |
7.58 |
19.7 |
0.54 |
19.16 |
32.01 |
33.67 |
|
3600 |
50.3 |
25.9 |
0.25 |
9.39 |
3.25 |
5.05 |
8.9 |
12.8 |
0.41 |
12.39 |
51.49 |
56.9 |
|
5400 |
50.2 |
26.8 |
0.21 |
9.38 |
3.24 |
5.04 |
8.89 |
11.5 |
0.36 |
11.14 |
53.39 |
61.28 |
|
7200 |
51.1 |
35.7 |
0.13 |
9.54 |
3.3 |
5.13 |
9.05 |
6 |
0.28 |
5.72 |
69.86 |
79.798 |
As it’s obvious from
the Table 1 the concentrates are enumerated in the order of increasing the amount
of impurities in their content. In the result of the conducted experiments on roasting
concentrates in laboratory conditions the following figures have been obtained:
according to sulphur oxidization degree (remaining sulphide sulphur) the concentrates
can be lined in the following sequence: 4,1,6,3,2,5,7; according to the desulphurization
degree the concentrates can be lined in the following sequence: 1,4,3,2,6,5,7;
according to the solvability degree the concentrates can be lined in the following
sequence: 1,4,6,5,3,2,7; according to the sulphur oxidization velocity the
concentrates can be lined in the following sequence: 1,2,3,5,4,6,7; according
to the desulphurization velocity the concentrates can be lined in the following
sequence: 1,4,2,3,6,5,7; according to the solvability velocity the concentrates
can be lined in the following sequence: 1,4,6,5,3,2,7.
Table 7 - Roasted
Product of Zinc Concentrate ¹6
|
Time, s |
Content,% |
Zn Solub., % |
Desulph.,% |
|||||||||
|
Zno |
Znê |
Znâ |
Fe |
Cu |
Pb |
SiO2 |
So |
SSO4 |
Ss |
|||
|
1800 |
50.4 |
16.4 |
0.37 |
8.12 |
3.27 |
5.53 |
9.36 |
18.8 |
0,31 |
18.5 |
32.54 |
21.99 |
|
3600 |
52.7 |
27.9 |
0.26 |
8.49 |
3.42 |
5.78 |
9.78 |
12.4 |
0,25 |
12.5 |
52.94 |
48.55 |
|
5400 |
54.3 |
37.3 |
0.32 |
8.75 |
3.52 |
5.95 |
10.08 |
7.2 |
0,3 |
6.9 |
68.69 |
70.12 |
|
7200 |
55.3 |
45.1 |
0.13 |
8.91 |
3.59 |
6.06 |
10.28 |
3.6 |
0,22 |
3.38 |
81.56 |
85.06 |
Table 8 - Roasted
Product of Zinc Concentrate ¹6
|
Time, s |
Content,% |
Zn Solub., % |
Desulph.,% |
|||||||||
|
Zno |
Znê |
Znâ |
Fe |
Cu |
Pb |
SiO2 |
So |
SSO4 |
Ss |
|||
|
1800 |
45.9 |
10.5 |
0.24 |
11.81 |
3.6 |
5.29 |
7.31 |
23.3 |
0.4 |
22.9 |
22.88 |
23.86 |
|
3600 |
46.3 |
7.9 |
0.23 |
11.92 |
3.63 |
5.33 |
7.38 |
23 |
0.33 |
22.6 |
17.06 |
24.84 |
|
5400 |
48.3 |
13.8 |
0.15 |
12.43 |
3.79 |
5.56 |
7.69 |
13.5 |
0.38 |
13.1 |
28.57 |
55.88 |
|
7200 |
49.2 |
25 |
0.14 |
12.66 |
3.86 |
5.67 |
7.84 |
13.3 |
0.37 |
12.9 |
50.81 |
56.54 |
The Concentrate ¹1 has
the best results of roasting quality when the Concentrate ¹7 has the worst ones.
That is regular and obvious as the Concentrate ¹1 has the least number of impurities
while the Concentrate ¹7 has the most.
According to the
influence of impurities on the quality factors one can come to the following
conclusions:
Impurities influence
differently on different quality factors.
The sum of Fe, Cu, Pb,
SiO2 with correlation rate -0.95 and Pb
with correlation rate -0.90 have the most
significant impact (regeneration) on sulphur oxidization velocity. Cu with correlation rate -0.51
has the least significant impact.
The sum
of Fe,
Cu, and Pb with correlation rate -0.84 and Fe with correlation rate -0.93
have the most significant impact (regeneration) on desulphurization
increase velocity. SiO2 with
correlation rate 0.19 has the least significant impact.
Table 9 – Zinc Concentrates Roasting Figures
|
Concentrate title |
Velocity |
Impurities content, % |
||||||||
|
Sulphur oxidization, gps*10-4 |
Desulphurization, %/s*10-2 |
Zn solubility, %/s *10-2 |
Fe |
Cu |
Pb |
SiO2 |
Sum |
|||
|
Fe,Cu |
Fe,Cu,Pb |
Fe,Cu, Pb, SiO2 |
||||||||
|
Con.¹1 |
4.3 |
1.3 |
1.21 |
6.2 |
0.28 |
0.61 |
2.6 |
6.48 |
7.09 |
9.69 |
|
Con.¹2 |
3.75 |
1.2 |
0.86 |
8.2 |
0.9 |
2.12 |
4.8 |
9.1 |
11.22 |
16.02 |
|
Con.¹3 |
3.63 |
1.2 |
0.94 |
7.1 |
4.1 |
2.9 |
4.4 |
11.2 |
14.1 |
18.5 |
|
Con.¹4 |
3.3 |
1.28 |
1.18 |
5.6 |
0.1 |
4.7 |
8.8 |
5.7 |
10.4 |
19.2 |
|
Con.¹5 |
3.33 |
1.11 |
0.97 |
7.8 |
2.7 |
4.2 |
7.4 |
10.5 |
14.7 |
22.1 |
|
Con.¹6 |
2.88 |
1.18 |
1.13 |
7.2 |
2.9 |
4.9 |
8.3 |
10.1 |
15 |
23.3 |
|
Con.¹7 |
2.46 |
0.78 |
0.7 |
10.5 |
3.2 |
4.7 |
6.5 |
13.7 |
18.4 |
24.9 |
|
Correlation rate of sulphur oxidization
velocity depending on impurities content |
-0.62 |
-0.51 |
-0.90 |
-0.72 |
-0.65 |
-0.88 |
-0.95 |
|||
|
Correlation rate of
desulphurization velocity change depending on impurities content |
-0.93 |
-0.54 |
-0.47 |
-0.19 |
-0.85 |
-0.84 |
-0.69 |
|||
|
Correlation rate of Zn solubility change depending on impurities
content |
-0.92 |
-0.53 |
-0.19 |
0.06 |
-0.84 |
-0.71 |
-0.48 |
|||
The sum
of Fe,
Cu, and Pb with correlation rate -0.84 ÷ -0,71
and Fe with correlation rate -0.92 have the most significant impact
(regeneration) on desulphurization increase velocity.
SiO2 with correlation rate -0.92 has the least
significant impact.
With the
potential difference of 0.069 eV at sulphide-oxide boundary, sulphur
oxidization, desulphurization and soluble compounds formation velocities will
be equal correspondingly: 4.3*10-4 gps, 1.3*10-2 %/s, 1.21*10-2
%/s.
With the
potential difference of 1.0195 eV at sulphide-oxide boundary, sulphur
oxidization, desulphurization and soluble compounds formation velocities will
be equal correspondingly: 2.46*10-4 gps, 0.78*10-2 %/s, 0.7*10-2
%/s.
If the potential
difference at sulphide-oxide boundary increases, sulphur oxidization, desulphurization
and soluble compounds formation velocities decrease.
Zinc sulphide concentrate which is a raw material in zinc
production and which undergoes roasting on the purpose mainly consists of heavy
metals sulphides. All the sulphides are semiconductors. Semiconductors in their
turn have positive or negative charges. If we roast a semiconductor with
negative charge, in the result we obtain an oxide with positive charges
prevailing. In the contact point of semiconductors with negative and positive charges
potential difference occur and double electrical layer that increases diffusion
resistance in roasting appears.
Calculation of Alternating Double Electrical Layer Value Depending on Impurities
Amount
Firstly, the conductivities
of concentrates and roasted products are determined. After that, we calculate the
Hall remainder of potential differences.
Having got the Hall potential differences value one can
calculate the Hall coefficients for each product.
VH= RjB0d (1)
Modifying the formula we get:
R= VH/(jB0d) (2)
Where B0= BM*χ/ε - magnetic induction in a semiconductor
sample, gauss;
BM – magnet magnetic induction, 800 gauss;
j – current density in a sample, à/sm2;
d – sample width, sm;
VH – Hall potential difference, volt;
R – Hall
coeficient;
ε – relative
magnetic permittivity (for sphalerite -7.9; for zinc oxide – 8.5);
χ - magnetic
susceptibility (for sphalerite 0.7*10-6; for zinc oxide – 0.33*10-6
ñì2/ã);
If we know Hall coefficient, we can determine the
concentration and charge sign in the semiconductor.
Hall coefficient for electrons:
R = -1/(e*n)
(3).
Hall coefficient for electron
vacancies:
R = 1/(e*p)
(4)
where e – electron charge,
1,602*10-19 Ê;
n – electrons
concentration in a semiconductor, 1/sm3;
p - electron vacancies concentration in a
semiconductor, 1/ñì3.
Thus, electrons concentration in a semiconductor equals:
n = -1/(e*R)
(5).
And electron vacancies
concentration in a semiconductor equals:
p = 1/(e*R)
(6).
The obtained calculation results are consolidated
in Table 2.5.
We calculate the contact potential
difference value according to the formula:
eUK= k0Tln(n/p)
(7).
When metal phases occur, semiconductors become
ligated. In this case contact potential difference can reach the
maximum value.
eUK = ∆Eg
(8).
Thus, when metal phases occur, contact potential difference
increases up to the forbidden band.
We calculate the forbidden
band value of concentrates and roasted products.
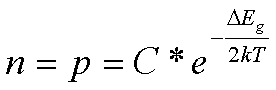 (9)
(9)
where k – Boltzmann constant, 0,86*10-4 ev/K.
Modifying the equation
(9) we get the following one for concentrates:
∆Eg = (ln C –
ln n)/(2kT) (10),
and for electron vacancies:
∆Eg = (ln C – ln p)/(2kT) (11).
To calculate the forbidden band we determine Ñ coefficient:
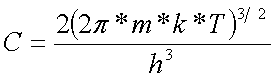 (12).
(12).
The obtained results are in the tables below:
Table 10 - Ñ coefficient
|
Temperature, Ê |
298 |
373 |
473 |
573 |
673 |
773 |
873 |
|
Ñ coefficient |
2.4E+37 |
3.3E+37 |
4.7E+37 |
6.3E+37 |
8.0E+37 |
9.8E+37 |
1.2E+38 |
|
Temperature, Ê |
973 |
1073 |
1173 |
1273 |
1373 |
1473 |
1573 |
|
Ñ coefficient |
1.4E+38 |
1.6E+38 |
1.8E+38 |
2.1E+38 |
2.3E+38 |
2.6E+38 |
2.8E+38 |
Cupric, ferric, lead, zinc sulphides as well as their oxides are semiconductors.
In the result of the research pyrite, copper pyrite and galena in low grade
zinc sulphide concentrates appeared to be semiconductors with electron
conductivity prevailing while their oxides after thermal treatment and
oxidization appeared to be semiconductors with electron vacancies conductivity.
So, in the contact point of semiconductors with electron and electron vacancy
conductivities contact potential difference occur. Metal phases, in their turn,
increase contact potential difference value as they are conductors with
electron conductivity.