SYNTHESIS AND STRUCTURE n-AMINOZILGLYKOZIDES
BY THE ï-AMINOACETOPHENON
1O.A. Nurkenov,
1A.S. Fazylov, 1Zh.S. Ahmetkarimova, 2R.A.
Ermuchanbetova,
1Zh.B.
Satpaeva, 3Zh.H. Muldahmetov
1Organic synthesis and coal chemistry
institute of Republic Kazakhstan,
2Kazakhstan
university of friendship of people
3Karaganda state technical
university
Now the market of
pharmaceutical production is presented to Republics Kazakhstans, basically, on
90 % import medical products that results not only to their significant dearly,
but also to direct dependence of deliveries from the countries-exporters. For
this reason, at session of 12-congress «Nur Otan» the President of Republic
Kazakhstan N.A.Nazarbaev has sounded the program of rise up to the end 2014ã. The pharmaceutical industry of
Kazakhstan and finishing of the market of production by own medical products up
to 50 % from their general number. The main stage of the successful decision of
this problem is full integration between many scientists, specializing in the
field of organic, bioorganic, pharmaceutical chemistry, pharmacology and
medicine. It is the important and multisector problem assumes the organization
new original, competitive in the market of domestic medical products which
development is conducted on results of fundamental scientific researches in the
field of thin organic synthesis, chemistry of natural connections, medicine,
technology, etc.
Many medical
products because of medicinal resistency of bacteria and viruses gradually lose
the efficiency [1,2]. Therefore constant development and introduction of new
medical products is an actual problem of a chemistry-pharmaceutical science.
One of perspective directions in search and creation
of new medical products is chemical transformation of molecules of known
bioactive materials, for example a method glycositical physiologically active connection on
carbohydrate center of sugars. Glycositical method usually result ins to
decrease in toxicity of substrate and increase in aqueous solubility of drugs.
Besides use of a method glycositical will allow to change permeability of
physiologically active connections through membranes, and attachment of these
connections to oligo-and to
polysaccharides will increase life expectancy of medical products [3].
As a result of search of paths
of the decision of this problem, employees of Institute of organic synthesis
and coal chemistry of Republic Kazakhstan earlier
have been carried out researches of interaction of some biogenic heterocyclic
amines with carbohydrates, biological activity of the synthesized connections
[4,5] is confirmed. With the purpose of continuation of these researches
studying reaction glucositical N-aminoacetophenon with
monosaccharoses is lead.
Synthesis
N-aminoglycosides carried
out the known classical method offered on Century by Sorokin in work [6],
direct condensation of initial amine with monosaccharoses in a spirit. As a
result of the executed researches of condensation of D-glucose, D-galactose and
L-arabinose with N- aminoacetophenon
it is positioned, that in the environment of absolute
ethanol at presence of 1-2 drops of an acetic acid (catalyst) it is possible to
achieve a satisfactory yield corresponding aminoglycosides (4-6):
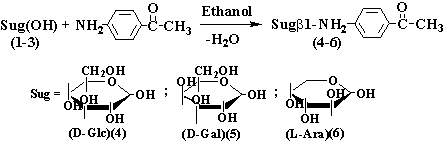
The
synthesized glycosides (4-6) are received with a yield of 55-74 %, represent
oils light-grow colors
sparingly soluble in polar solvents.
In NMR
spectra N- aminoglycosides
(4-6) it is necessary to note presence of a wide
intensive strip in the field of 3405-3520 sm-1, corresponding valence
vibrations of hydroxyl groups (IT) of carbohydrates. Presence of several peaks
in the field of 1010-1080 sm-1 testify about piranozes to the form glycosidesthe rest.
At the
analysis of a spectrum of a nuclear magnetic resonance-1Í (DMSO-d6,
500 MHz) connections (4-6) is positioned, that signals of protons ÑÍ-, ÑÍ2- groups a
glycosic part of a molecule are displayed in the field of 3,30-3,60 ì.d. In the
form of a complex multiplet. Anomers proton Í (1) carbohydrate rests is
displayed by a triplet at 4,31 ì.d. With ÊÑÑÂ = 7,9 Hz, characteristic for
b-anomer and
testifying to interaction anomer protons not only with the proton next a
trance-axial at Ñ2,
but also with amines proton
NH, a leaving doublet at 7,1 ì.d. Signals of three protons of
secondary it-groups of a carbohydrate skeleton are displayed by three doublets
at 4,67, 4,7 both 4,73 ì.d.
And a proton primary it-group a triplet at 4,52 ì.d. With ÊÑÑÂ = 5,8 Hz. Methyl protons
piperazines cycle are displayed in the form of a singlet in the field of 2,4 ì.d. In the
field of 6,71-7,75 ì.d.
Signals protons a
benzene ring are marked. The integrated curve corresponds to total of protons.
The table.
Physical and chemical constants and data of the element analysis of the
synthesized connections (4-6)
|
Connection |
Yield % |
Melting point, 0C |
It is found, % |
The formula |
It is calculated, % |
||||
|
C |
H |
N |
C |
H |
N |
||||
|
4 |
55 |
225 |
59,93 |
9,85 |
3,88 |
C18H35NO6 |
59,81 |
9,76 |
3,87 |
|
5 |
70 |
166 |
59,93 |
9,85 |
3,88 |
C18H35NO6 |
59,81 |
9,76 |
3,87 |
|
6 |
74 |
177 |
61,02 |
9,55 |
4,69 |
C16H29NO5 |
60,93 |
9,27 |
4,44 |
Thus,
condensation of monosaccharoses of D–glucose,
L–arabinose and D-galactose with N-aminiacetophenon us for
the first time are synthesized and characterized new N-aminoglycosides (4-6), of
interest as potential bioactive connections. Composition and a structure new
glycopiranozilamines are
confirmed by data of the element analysis and IR-spectroscopy.
Experimental
part
IR-spectra
of connections (4-6) are removed on a spectrometer from the Fourier-converter
«AVATAR-320» firms NICOLET in tablets with KBr. Melting points are certain on
device " Boetius ". The control over a course of reaction and
cleanliness of the received connections carried out a method shallow layer òîíêîñëîéíîé hrommotagraphy on
plates «Silufol
UV-254» in system isopropyl alcohol-ammonia-water - 7:2:1. A plate displayed in
pairs iodine.
N-(-4-acetyphenyl)-b-D-glucopiranozilamine (4).
Of 1,21g (0,01 mole) n-aminoacetophenon and 1,8g of
D-glucose in 20 ml of absolute ethyl alcohol agitated a mix at temperature
55-600Ñ within
8 hours, have added pair drops of a cold acetic acid. Reactionary weight
maintained within day in a freezing case at temperature-180Ñ, formed
jelly the rest
washed out some times hexane. 1,52g of a derivant piranoziamin (4) in
the form of a powder of beige color are received. A yield of 55 %. It is found,
%: C 59,93; H 9,85; N 3,88. C18H35NO6.
It is calculated, %: C 59,81; H 9,76; N 3,87. NMR 1Í, ì.d., J/Hz: 2,25 with (3Í, ÑÍ3-Àr); 3,30-3,60
m (6Í, (Í2-Í6)); 4,31 t (1Í, Í (1), J-7,9); 6,71 d (1Í, NH, J=7,9); 7,21 d (1Í, Í-Àr-N, J=7,6); 7,75 with (1Í, Í-Àr-ÑÍ3).
N-(-4-acetyphenyl)-b-D-galactopiranozilamine
(5) it is received similarly (4). A yield of 70 %. It is
found, %: C 59,93; H 9,85; N 3,88. C18H35NO6.
It is calculated, %: C 59,81; H 9,76; N 3,87. NMR 1Í, ì.d., J/Hz: 2,25 with (3Í, ÑÍ3-Àr); 3,30-3,60
m (6Í, (Í2-Í6)); 4,31 t (1Í, Í (1), J-7,8); 6,71 d
(1Í, NH, J=7,8); 7,21 d
(1Í, Í-Àr-N, J=7,6); 7,75 with (1Í, Í-Àr-ÑÍ3).
N-(-4-acetyphenyl)-b-L-arobinopiranozilamine (6).
A yield of 74 %. It is found, %: C 61,02; H 9,55; N 4,69. C16H29NO5.
It is calculated, %: C 60,93; H 9,27; N 4,44. NMR 1Í, ì.d., J/Hz: 2,35 with (3Í, ÑÍ3-Àr); 3,08-3,67
m (6Í, (Í2-Í6)); 4,56 t (1Í, Í (1), J-7,3); 6,25 d
(1Í, NH, J=7,3); 6,81 d (1Í, Í-Àr-N, J=7,1); 7,15 with (1Í, Í-Àr-ÑÍ3).
The list of the used sources
1.
Tulegenova A.U., Muminov T.A., Praliev K.D. Search of new antitubercular
drugs - the essential requirement of modern phthisiology // The bulletin Kazakh
state. -1998.- ¹1.- P.14-22.
2.
Veselov A.J., Krasnikova E.I., Çàâàäîâñêèé I.M.medicinal
resistency ìèêîáàêòåðèé a
tuberculosis // Antibiotics and chemotherapy.-1992.- ¹3.- P.13-15.
3.
Kulakov I.V., Nurkenov O.A., Ilin A.I., Kulmanov M.E. N-glycosidamines:
methods of synthesis, a structure and biological activity. Karaganda, 2010.
156.p.
4.
Ibraev M.K. Synthesis, a structure and biological
activity new multifunctional derivativ some natural both synthetic
1,2-aminoalcohols and amino acids: avtoref.
doct.chem.: 02.00.04. – Karaganda, 2007. 45 p.
5.
Ainabaev A.A. Synthesis, transformations and
biological activity of sulfur-containing multifunctional derivatives of the
some alkaloids: avtoref. cand.chem.sci.: 28.10.07. - Karaganda: ÈÎÑÓ ÐÊ, 2007. - 22ñ.
6.
Mashkovskii M.D. Medical
product,15 edition.- Ì.:«New wave», 2007. 1206 p.
