Ïóáëèêàöèè â “Nauka i studia”( Przemysl,
Ïîëüøà) èëè â ×åõèè
Ñåêöèÿ “Áèîëîãè÷åñêèå íàóêè/Áèîõèìèÿ è
áèîôèçèêà” èëè
“Ìåäèöèíà/Ýêñïåðèìåíòàëüíàÿ ôàðìàêîëîãèÿ”èëè
“Õèìèÿ/Õèìèêî-ôàðìàöåâòè÷åñêîå
ïðîèçâîäñòâî”
Mamina Î.Î., Kovalenko Z.I.
Ukrainian National University of Pharmacy,t.Kharkov
Identification of synthetic food dye Allura Red by HPLC
Allura
Red, disodium 2-hydroxy-1-(2-methoxy-5-methyl-4-sulfonato- phenylazo)-naphtalene-6-sulfonate
is synthetic food dye, E 129 according to the
international enumeration system of food additives [1].
Allura
Red widely used in
food, pharmaceutical and cosmetic industries, but chronic use causes allergies,
disorders of biochemical reactions and cancer [1,2]. This dye affect the chromosomes and violates the division
of cells in the tissues [2].
To determine the synthetic food dyes uses such analytical methods, as
chromatographic (HPLC, TLC, electrophoresis) and spectral (UV and visible
spectrophotometry)[1,3]. HPLC-methods are characterized
by various chromatograph conditions, depending on individual properties of food
dyes. The purpose of work - the identification of Allura Red when using unified
conditions HPLC suitable for studies of pharmaceuticals, food products,
biological objects.
For research standards
have been used «Dyes analytical
standart kit 10 pcs», Institute of
dyes and organic products (IBPO),
Poland.
The chromatographic process of Allura Red has been conducted on microcolumn liquid chromatograph «Milichrom À-02» on base research union «Ànalitika» in reversed-phase variant with using of
metalic column with non-polar absorbent Prontosil 120-5C 18 AQ, 5 μm.
Allura Red has been eluted with mobile phase in the mode of linear gradient – from eluent À (5 % acetonitrile and 95% buffer solution - 0,2 Ì solution of lithium perchlorate in 0,005 Ì solution perchloric acid) to eluent B (100% acetonitrile) as during 40 min. Regeneration of column has been conducted during 2 min with mixture of solvents.The flow rate of the mobile phase has been formed 100 μl/min, injection volum – 1 μl. The detection of of Allura Red has been conducted by UV- detector at 7 wavelengths: 210, 220, 240, 250, 260, 280, 350 nm. The optimal values of column
temperature
– 40°Ñ and pressure of pump – 4,2 MPa.
mV
The symmetry factor (As)
of peaks
(1,52 ± 0,03) have been received in result of the
chromatographic process of Allura Red with using unified conditions HPLC. The identification of of Allura Red has been conducted with using absolute parameters of retention time (tR) and retention volum (VR), and spectral relations, which determined
as ratio of values of absorbance at wavelengths - 220-350 nm – to values of absorbance at 210 nm (Fig.,Tab.).
Time¸
min
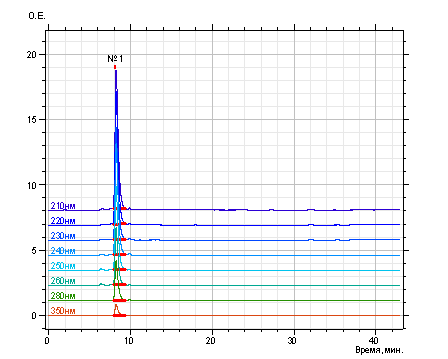
Fig. Chromatogram
of Allura Red (aqueous solution concentration
8,5 mg / ml)
Table
Retention
parameters and spectral relations (n = 5)
|
Allura Red |
Retention parameters |
Factor of capacity, k´ |
||||
|
tR, min |
VR, μl |
|||||
|
8,21
± 0,02 |
821,1 |
4,47 |
||||
|
Spectral relations |
||||||
|
À220 nm
|
À240 nm
|
À250 nm
|
À260 nm
|
À280 nm
|
À 210 nm |
|
|
0,915 |
0,761 |
0,567 |
0,386 |
0,265 |
0,072 |
|
Results of research can be recommended for the
analysis of pharmaceuticals, food and biological
objects on synthetic food dyes.
Conclusions
1.
The HPLC-study of Allura Red with the use
of a unified conditions of analysis suitable for studies of pharmaceuticals, food
products, biological objects was conducted.
2. The basic parameters of identification of Allura Red have been defined in terms of analysis.
Literature
1. Bolotov V.M. Food dyes: classification, properties,
analysis, application/ V.M. Bolotov, À.P. Nechaev, L.À.Sarafanova.-
SPb: Publishing
house GIORD, 2008. - 240 P.
2. Pesnya D. S. Study of toxic and genotoxic effects
of synthetic food dyes method Allium test / D. S. Pesnya, A. S. Romanovsky, I.
M. Prokhorov. // The Yaroslavl pedagogical Bulletin - 2012 - ¹ 3 – Vol. III (Natural Sciences)- pp.86-93.
3. Kiseleva M. G. Optimization of Conditions for the HPLC
Determination of Synthetic Dyes in Food / M.
G. Kiseleva, V.V. Pimenova, K. I. Eller//J.
of Anal. Chem. – 2003. - Vol. 58, No. 7. - pp. 685–690.