ì.ò.í.
Àóáàêèðîâ À.Ì.
Èííîâàöèîííûé Åâðàçèéñêèé óíèâåðñèòåò, Êàçàõñòàí
IMPROVEMENT OF FILTERING TECHNOLOGIES AND THE CRYSTALLIZATION OF SODIUM
HYDROALUMINATE IN ALUMINIUM INDUSTRY
In recent times, due to the usage of bauxites
from new deposits in the aluminium industry
the impurity composition of aluminate solutions has changed. This
reflects negatively on the several processing stages of production,
particularly on the division producing rough gallium. Another reason
prejudicial to the production of gallium is reduction of its content in the
bauxites, as well as the loss of valuable rare metal in the derivation with
ferruginous sands. This implies a decrease in the concentration of gallium in
the aluminate solutions.
Emerging
issues require further study and the development and implementation of new
technological schemes.

Figure 1- Sodium hydroaluminate production unit:
ÊÐ1-K8 - Crystallizer of sodium hydroaluminate;
ÃÖ1,2 -
hydrocyclone; ÏÃ –thickening repulpator; ÑÃ - thickener; ÌÏ – mixer of pulp of
filter power, ô1-3 – vacuum filter, Ð1,2-receiver, HB1,2 - vacuum pump, H1-H12
–Centrifugal pump, Ìô1,2 – filtrate mixer, ÌÀ1,2 – aluminate solution mixer.
Direct extraction from solutions by cooling
became impractical; therefore a new technological scheme of processing of
working solutions with obtaining artificial solutions rich in gallium has been
developed. Weak working solutions and drainage of sodium sedimentation
tank are cleared at the beginning of chlorides, sulfates and carbonates by
evaporation of these solutions with concentration of 220 g/l to concentration
of 320 g/l with all sodium salts precipitate. In
order to achieve a concentration of 150-300 g/l in the thickened pulp, the
evaporation and thickening should be carried out so as much as possible
chlorides precipitate. Draining of the thickener is directed to the allocation
of CGC by re-evaporation of the solution to the concentration of 420 g/l: in
this case the solutions become sodium aluminate saturated, which precipitate as
sodium hydroaluminate to Na2O · Al2O3 · 2,5H2O and subsequently release:
- due
to cooling of the solution after the second stage of evaporation from 140 to
85-90 C;
- soaking for 10-14 hours;
-
afterfiltration on filters BOU.
At the same time sodium hydroaluminate precipitates with about 25%
moisture, which dissolves in water and has a caustic ratio of about 1,6 units
and the concentration of about 160 g/l, which is pumped to the sintering
workshop as aluminate solution at desiliconization stage, followed by extraction
of alumina by means of decomposition, and cleaned filtrate of sodium
hydroaluminate is directed to the extraction stage of CGC, which is carried out
by salting out with sodium chloride, thickened pulp from the first evaporation
stage is used as a chloride primer which is supplied with ratio of chlorine of
thickened pulp to Al2O3 of filtrate of about 0,4 – 0,7.
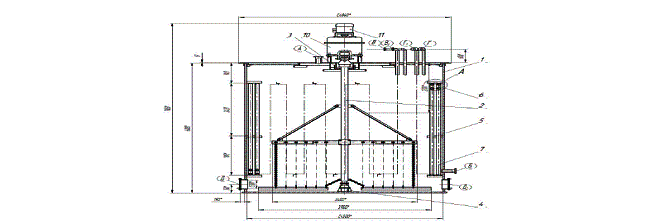
Figure 2 – crystallizer:
1 – vat; 2 - mixer; 3 -
hydraulic lock; 4 - step-bearing; 5,6,7 –coil; 8 –clamp; 9 – bracket; 10 –
reducer; 11 - electric motor
The evaporated solution from the mixer with concentration of Na2Oky -
440 g/l, sodium percentage - 2.8
÷ 3.0 and αky - 2,9 ÷ 3,0 in the amount of 15.4 m3/h is directed to the
crystallizers of sodium hydroaluminate with PB pumps. Crystallizers operate in
batch mode, each is receiving and consumable by turns. Solution in the
crystallizers is stirred with mixer driven by electric motor through reducer
SPC-45. The temperature of the solution supplied to the crystallization is of
105-115 0C. Crystallization is carried out until the caustic ratio of liquid
phase of the pulp reaches 8.0 - 9.0 for 10-14 hours, with the pulp cooled to
80-85 0C which is achieved by a pair of registers which are fed with industrial
water. In order to achieve the intended caustic ratio of liquid phase of the
pulp of sodium hydroaluminate, the concentration of feed solution should not be
less than 435 - 445 g/l Na2Oky.
Received pulp of sodium hydroaluminate in the amount of 14,5 m3/h is
pumped into the mixer and supplied to the filters BOU with 20 pumps. The pulp
from the crystallizer is pumped is not in full, 1 m of the pulp is left for
priming next crystallization of sodium hydroaluminate. This method of supplying
the primer to the process is less appropriate because redistribution of sodium
hydroaluminate according to granulometric composition may occur during
unloading the crystallizer, in this case the largest crystals which are the
products can be found at the bottom of the crystallizer and are used as the
primer while the smallest ones are carried in the first place and sent to the
next processing stage. The condition of the primer surface is very important in
the crystallization process. The best primer is the primer with active surface.
The smallest particles of sodium hydroaluminate have more active surface.
Besides, the process of crystallization is heterophase since it consists of at
least two phases. During heterophase processes where the main stage runs at the
interface, the process speed depends on the total surface area that is on the
specific surface area of supplied primer. When using smaller primer the total
surface area where crystallization takes place increases significantly which
will have an immediate effect on the overall speed of the process.
Complete unloading of the crystallizer with further classification of
the obtained precipitate of sodium hydroaluminate, after which the finer
fraction is returned back as the primer in the crystallizer and the coarser one
is sent further to the next processing stage can improve the characteristics of
crystallization and filtration of the obtained precipitate.
List of
references:
1) Shalavina Ye.D., Romanov G.A.,
Yevseyev Yu.N. Obtaining gallium
from aluminate solutions - Alma-Ata: Nauka, 1990. – p. 204
2) Yeremin N.I. Gallium - M., 1964.
3) Kassatkin A.G. Basic processes and
devices of chemical technology. Publishing
house "Chemistry", Moscow, 1971.
4) A.I. Ivanov, G.N. Kozhevnikov. Complex processing of bauxites. Yekaterinburg, 2003.
5) Utkin N.I. Production of non-ferrous metals. - 2nd ed. - M.: Intermet Engineering, 2004. - p.
442.
6) Ibragimov A.T., Budon S.V.
Development of the technology of alumina production from bauxites in
Kazakhstan. Pavlodar. 2010.