Berillo D.A., Turmukhanova
M.Zh., Ahmedova Sh. S.
Al-Faraby Kazakh National
University, Almaty, Kazakhstan
Synthesis and biological activity of iminoethers of
2-methyl-2-aminoderivatives of propane acid
α-aminonitriles are suitable syntones for introduction of various
functional groups which allow enlarging the range of potentially biologically
active nitrogen containing organic compounds.
In this work the result on synthesis and biological activity of
iminoethers of 2-methyl-2-aminoderivatives of propane acid are reported. We
have synthesized α-aminonitriles of heterocyclic, aromatic and aliphatic
amines and their derivatives (codes 1a-d) and screening for some kinds of
biological activity has been carried out.
The results of primary biological screening of
N-isobutyronitrilpiperidine and its derivatives I (a-c) are presented in tables
1-4.
Table 1 – acute toxicity of compounds I (a-d)
when being introduced intraperitoneously to white mice.
|
The name of
compounds |
LD50
when being introduced
intraperitoneously to white mice, mg\êg |
|
amidoxime 2-methyl-2-N-piperidylpropane acid (I à) |
300 |
|
amid succinate of
2-methyl-2-N-piperidylpropane acid (I b) |
519,4+21,48 |
|
2-methyl-2-N-piperidylpropane
acid (I c) |
100 |
|
Nitrile hydrochloride
2-methyl-2-N-piperidylpropane acid (1 d) |
100 |
|
streptomycin |
213,8 +22,61 |
|
Klemastine |
154,0+12,1 |
|
Lidocaine |
95,0+13,1 |
It follows from the data in Table 1 that amidoxime (Ia) and amid
succinate of 2-methyl-2-N-piperidylpropane acid (Ib) have low toxicity, while
2-methyl-2-N-piperidylpropane acid (Ic) and nitrile hydrochloride of
2-methyl-2-N-piperidylpro-pane acid (Id) have the same toxicity comparable to
preparation Lidocaine which is widely used in medical practice.
Table 2 – Spasmolitic activity of nitrile
derivatives of 2-methyl-2-N-piperidyl-propane acid I (a-d) of all tested
compounds, nitrile hydrochloride of 2-methyl-2-N-piperidylpropane acid (Id)
revealed activity.
|
Index of compounds |
Change of
length of intestines after compound administration |
Activity
by acetylcholine a spasm (1 mg
acetylcholine / 1 mL of medium) |
Activity by
histamine a spasm (1 mL histamine /1
mL medium) |
|
I à |
Increase 1mm |
Decrease. 3mm |
Increase 1mm |
|
I b |
Increase 2mm |
Decrease. 2mm |
Decrease. 4mm |
|
I ñ |
Increase 4mm |
Increase. 2mm |
Decrease. 4mm |
|
I d |
Ñîêð. 1mm |
0 |
0 |
|
“No-shpa” |
0 |
0 |
0 |
|
Klemastine |
+1 |
+1 |
+1 |
|
Acetylcholine |
- |
4,0 |
- |
|
Histamine |
- |
- |
4,0 |
Table 3 Antibacterial activity of nitrile
2-methyl-2-N-piperidylpropane acid (Id) and streptomycine after 24 hours of
incubation in Beal-peptone broth (the growth of bacteria when diluted 1/100)
|
The name of
compounds |
Escherichia coli |
Sal.chol.suis |
Salmonella typhymurium |
Staphylococcus aureus |
|
I d |
2 |
2 |
2 |
2 |
|
streptomycin |
0 |
0 |
0 |
0 |
|
MPB (Control
crops without introduction antibacterial compounds) |
4 |
4 |
4 |
4 |
Nitrile hydrochloride 2-methyl-2-N-piperidylpropane acid (Id) reveals
antibacterial activity, however, by the expressiveness of the effect, the
activity of compound (Id) is not comparable to the preparation of comparison
streptomycin [1].
Table 4 Analgesic activity of
nitrile hydrochloride of 2-methyl-2-N-piperidylpropane acid (Id) on the model
“Tail-flick”.
|
Index of compounds |
Speed
of approach of effect |
Duration of analgesia |
Duration of full analgesia |
|
I d |
5 minutes |
165,0+11,2 |
45,3+7,1 |
|
Tramal |
5 minutes |
75,0+9,1 |
- |
Compound (Id) possesses a spasmolytic and moderate antibacterial
activity as well as a marked analgesic activity and authentically surpasses
Tramal having shown a higher analgetic activity than Tramal. It causes a
complete analgesia and, hence, has a greater range of pharmacological action
[1].
Thus, it is stated that nitrile hydrochloride of 2-methyl-2-N-piperidylpropane
acid (Id) (Scheme 1) has shown a high analgesic activity, moderate spasmolitic
and antimicrobial activity, however, this compound is highly toxic, this being
an unfavorable factor for practical use, while 2-methyl-2-N-piperidylpropane
acid (Id) its amidoxime and amide possess all the above mentioned properties of
N-isobutyronitrilepiperidine, except high toxicity. The investigation on the
biological activity of nitrile derivatives of 2-methyl-2-N-piperidylpropane
acid have shown that in going from nitrile to its derivatives. Toxicity of
substances decreases in the series acid > amidoxime > amide.
In this connection, with the aim of searching for new potentially
biologically active compounds we have synthesized a number of iminoethers of
2-methyl-2-N-aminoderivatives of propane acid (6-10) from the corresponding
aminonitriles according to the scheme given below.
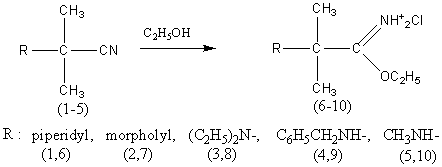
The structure of the obtained compounds (1-10) is verified by the data
of physico-chemical methods of analysis.
Reference
[1] ¹22043 The innovative patent R.K. D.A. Berillo, Sh.S. Ahmedova, S.N.
Shin, O.A. Berillo Nitrile 2-methyl-2-N-piperidylpropane acid possessing a
spasmolitic, analgetic and antimicrobial activity. (15.12.2009), bulletin ¹12.