Ph.D., associate Professor Lydmila
Ignatovich
Belarussian State University
of Technology, Belarus.
Ph.D., associate Professor Lydmila
Dubovskaya
Belarusian State Academy of
Arts, Belarus.
Influence
of the interaction of hardener and mineral binding THE STRENGTH OF COMPOSITE
Introduction. It is known that
the waste from wood processing industries often burned, but the calorific value
of the sawdust is low enough and the use of soft waste in this capacity is the
least economically expedient in relation to other types of wood fuel. There is
more rational use of waste wood - as a raw material for the production of
composite materials in the mineral binder. A good mineral binder is a liquid
glass, which has high adhesion to wood, low cost and availability of raw materials.
Significant disadvantages of liquid glass are its low water resistance. Improve
the adhesive properties of sodium silicate and water resistance can be achieved
by modifying hexafluorosilicate sodium, which also accelerates the process of
setting and hardening of the liquid glass.
The main part. In order to
identify the reaction products hexafluorosilicate sodium silicate in the
presence of sawdust and without electron microscopic analysis were and IR
spectroscopic studies of the initial components and products of their
interaction. Infrared absorption spectra of the test compounds were recorded in
the frequency range of 400-4000 cm-1 by direct analysis of individual
substances without mixing with KBr. Spectra were obtained with single-beam
FT-IR spectrometer "FTIR-8601 PC". In the analysis we recorded the IR
absorption spectra of the compounds considered in the general case, the number
of absorption bands related to the vibrations of the ion or molecule, and the
frequency range of their occurrence. The results of these studies suggest that
the basis of the interaction of the starting compounds (water glass and
Na2SiF6) is a chemical reaction oc-Penitent by equation 1:
2(Na2O·3,2SiO2∙nН2О) +2Na2SiF6
® 8NaF + SiF4↑+
7,4(SiO2∙nН2О) (1)
The
reaction of proceeds sufficiently quickly (within 10-15 minutes) and
irreversible. Since the content of NaF in the solid product interaction (TPR)
Na2SiF6 and Na2SiO3 24 hours was the same as after the 10-minute interaction,
the solid product and the parent compounds were analyzed for silicon content by
electron microscopy analysis using scanning microscope with a microprobe, which
allows to determine the chemical composition of the element by element of the
objects. It was found that the total of the total silicon in the initial
product (Na2SiF6) + (Na2SiO3) is 25.28 wt.%. This exceeds the total silicon
content in the solid product interactions on the value of 12.39 wt.%. This
difference corresponds exactly to the total silicon content in the gaseous
product of interaction. The above scheme can be balance (Equation 2):
(Si Na2SiF6+
Si Na2SiO3) - Si = Si SiF4 (2)
Or,
based on 100 wt. h of liquid glass and 16 wt. h hexafluorosilicate sodium
balance in distribution of silicon is (Equation 3):
(5,51 +
2,38) – 6,7 = 1,20 wt. % (3)
The
presence of silica in the solid product is confirmed by the interaction of the
initial components of IR spectroscopic studies. IR spectra were analyzed
products are shown in Figure 1. Confirmation that the mixture and Na2SiO3
Na2SiF6 in the presence of H 2 O is the reaction of interaction, leading to
destruction and SiF62-SiO32-anions to form SiO2, is the absence in the IR
spectrum of the mixture Na2SiF6 Na2SiO3 absorption bands corresponding to the
vibrations of the anion SiF62--733, 494 and 475 cm and SiO32-1018, 900, 770,
474, 436 cm (Fig. 1). View of the IR spectrum of a mixture Na2SiF6 Na2SiO3
after the interaction is almost identical to the IR spectrum of amorphous SiO2.
The absence in the IR spectrum of the mixture and Na2SiO3 Na2SiF6 absorption
bands corresponding to the vibrations of NaF, due to the fact that its content
of impurities NaF (equation) below the lower limit of detection of its
appropriate instruments [1].
The
study results assume that the adhesion of the particles of wood composite [2]
due to the formation of a stable crystal structure (Fig. 2), consisting of
molecules of SiO2 ∙ 2H2O and NaF, formed by the interaction of sodium
hexafluorosilicate (Na2SiF6) and sodium silicate (Na2SiO3) for the above-given
reaction. At the same time, the IR spectra of composite materials designed
purpose [2] found the absorption bands corresponding to vibrations of the anion
SiF62-730, 490 and 474 cm (Figure 2). This indicates the presence of sodium
hexafluorosilicate in the resulting materials. Above will most likely reaction
of Na2SiO3 Na2SiF6 and adding filler - sawdust - occurs is not completely due
to possible steric hindrance arising from the interaction of components and
wood bonding with each other [3].
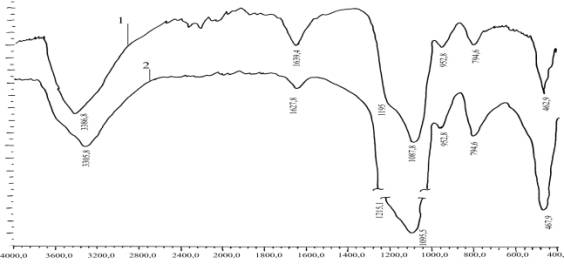
Fig. 1
IR absorption spectra1 2Na2O • SiO2 · nH2O + 2 Na2SiF6;
2 - amorphous SiO2
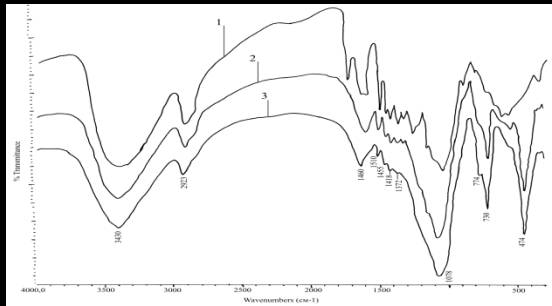
Fig. 2
IR absorption spectra1 sawdust, 2 - the material of construction of 3 -
insulating material
Conclusion. Improving the strength of
materials produced by small pieces of wood and water glass in the presence of
sodium hexafluorosilicate, due to the formationnew hydrogen bonds with the wood
due to their participation in shaping the structure of fluoride ions present in
the anion SiF62-and have greater electronegativity. [4]
Increase
the water resistance of such materials sposobstvovuet reduction of hydrophilic
hydroxyl groups, clearly visible when comparing the IR spectra of the
"Silk-K", "Silk-E" and the original wood (wood of the
absorption band of the 3000 - 3700 cm-1 is much deeper and wider ). [3]
Literature
1. Атлас инфракрасных спектров поглощения
и рентгенографических данных комплексных фторидов металлов IV и V групп / Р.Л.
Давидович [и др.]; под общ. ред. Р.Л. Давидовича. – М.: Издательство, 1972. – 252
с.
2.
Дубовская, Л.Ю. Композиционный материал на основе древесных отходов и
минерального вяжущего / Л.Ю. Дубовская, А.А. Янушкевич // Известия Белорусской
инженерной академии. – 2004 – №2. – С.
29 – 30.
3. Карклинь В.Б. ИК-спектроскопия
древесины и её компонентов / В.Б. Карклинь, Е.Э. Охерина // Химия древесины. –
1981. – №4. – С. 38 – 44.
4. Жбанков, Р.Г. Инфракрасные спектры
целлюлозы и её производных / Р.Г. Жбанков. – Минск, 1964. – 338 с.