UDC
541.124.2
N.R. BUKEIKHANOV2, P.B. VOROBYEV1, I.M. CHMIR’2, L.V. LI1,
A.S. ZULKASHEVA1,
T.P. MIKHAILOVSKAYA1
1JSC «Institute of chemical sciences named after
A.B. Bekturov», Almaty
2FSBEI HPE Moscow State Technological University
“STANKIN”,
Russian Federation, Moscow
CONJUGATED AND COMBINED CHEMICAL PROCESSES
Abstract. Ñombined chemical
reactions include such leading industrial processes as oxidative chlorination,
oxidative ammonolysis, oxidative esterification whereby modern bulk products -
vinyl chloride, acrylonitrile, and terephthalo- and isophthalonitrile,
vinyl acetate are produced. Such well-known polymeric materials as polyvinyl
chloride (manufacture of double-glazing windows, linoleum, packaging
materials), polyacrylonitrile (manufacture of "nitrone" fiber, carbon
fiber-reinforced plastic), polyesters, polyamides, polyurethanes (synthetic
fibers, rubbers, adhesives), polyvinyl acetate (PVA building adhesive, the starting
material for the polyvinyl alcohol used to prepare the high modulus fibers and
others) are obtained from these monomers. A number of processes based on the
coupled reactions such as oxidative ammonolysis, oxidative dealkylation, producing
of terephthalic acid by liquid phase oxidation of p-xylene have been developed in
Kazakhstan under the supervision of academician of NAS RK - B.V. Suvorov.
Academician Boris Viktorovich Suvorov defined a
coupling as a key step of the complex reactions’ mechanism [1-3]. Consistently [4,
5], acceleration (and deceleration) reactions by combining two or more
components in a reaction mixture are typical for the conjugated complex
reaction mechanism. Suvorov and co-workers [6] proposed a methodological
procedure of defining conjugation stages in complex catalytic process, which lies
in a proof of the transfer of starting material fragments from one direction of
chemical process to another parallel direction by identifying the corresponding
reaction products. Thus, the transfer of fragments was detected by using heteroatomic
compounds as starting materials. For example, cyanopyridines are formed in small amounts in the process
of oxidation of methylpyridine (picoline) on the vanadium oxide catalysts,
along with the products of partial oxidation - pyridine carboxylic acid and pyridine
aldehyde. The efficiency of donor nitrogen (nitrogen-containing fragments) transfer
was determined by calculation of the experimental data of catalytic oxidation
of alkylpyridines by equation S = (A * 100) / [100 - (B + C)] where: S – selectivity
of nitrile formation, in%, A - nitrile
yield, in %, from the theoretically possible, B - the amount of unreacted
starting material, C - total yield of the defined reaction products, in%, from the
theoretically possible. According to calculations the selectivity of cyanopyridine
formation can reach 81% in the process of 2-methylpyridine oxidation [6].
Studying kinetics of heterogeneous catalytic
oxidation of 4-methylpyridine into isonicotinic acid, the raw material for
antituberculous drugs, it was able to detect the presence of coupling between
the stages of pyridine-4-aldehyde formation and deep oxidation of raw and desired
reaction product, carrying with nitrogen-containing fragments, which play a
role of the nitrogen donor for cyano
group formation [7]. The kinetic model of this reaction, carrying in the flow
integral reactor, represents a system of ordinary differential equations,
numerical solution of which was performed by the Gear method [8]:
-dC4-Pic/dt = (k1
+ k4 ) · C4-Pic;
dC4-PyCHO /dt = k1 · C4-Pic - (k2 + k6) · C4-PyCHO;
dC4-PyCOOH /dt = k2
· C4-PyCHO - (k3 + k5) · C4-PyCOOH ;
dCPy /dt = k3 ·
C4-PyCOOH;
dCCO2 /dt = k4
· C4-Pic + k5 · C4-PyCOOH;
dC4-PyCN /dt = k6 ·
C4-PyCHO.
The optimum values of kinetic stages’
constants were defined from the conditions of minimizing the sum of squared
deviations between experimental and calculated reagents’ concentrations by
using Powell’s method [9]. The standard deviation for each product does not
exceed 8%. Table 1 shows the calculated kinetic stages’ constants (kj) of
4-picoline oxidation over the V-Ti-Zr-oxide catalyst at the temperature of 245îÑ and different ways
of water supply into the contact area. The observed increase of the kinetic
stages’ constants of initial 4-picoline and intermediate isonicotinic aldehyde conversion
on increasing concentration of water vapor in the reaction mixture, presumably,
is connected with the increase in the surface catalyst acidity and enhancement
of its adsorption ability with respect to pyridine derivatives. It is known
that Bronsted acidic sites are formed as a result of the dissociative
adsorption of water molecules on the surface of oxide catalysts [10].
Table 1 - Influence of water
vapor concentration in the contact area on the kinetic stages’ constants of
4-picoline oxidation over the V-Ti-Zr-oxide catalyst at a temperature of 245îÑ. The initial
concentrations: [4-picoline] = 1,1·10-4,
[O2] = 9,4·10-3 mol·L-1.
|
Stages
|
|
kj , ñ-1 |
|||||
|
Mechanism
stages |
[H2O] ·103, mol·L-1 |
||||||
|
|
0 |
1,3 |
2,45 |
5,1 |
8,9 |
15,2 |
|
|
1 |
4-Pic ® 4-PyCHO |
1,788 |
1,970 |
2,799 |
3,891 |
4,269 |
4,241 |
|
2 |
4-PyCHO ® 4-PyCOOH |
1,713 |
1,968 |
3,790 |
6,249 |
6,389 |
9,571 |
|
3 |
4-PyCOOH ® Py |
0,290 |
0,126 |
0,080 |
0,088 |
0,078 |
0,056 |
|
4 |
4-Pic ® CO2 + NOx |
0,084 |
0,276 |
0,485 |
0,316 |
0,118 |
0,161 |
|
5 |
4-PyCOOH®CO2+ NOx |
0 |
0 |
0 |
0,667 |
0,434 |
0,514 |
|
6 |
4-PyCHO ® 4-PyCN |
0,164 |
0,597 |
0,814 |
1,079 |
0,862 |
0,650 |
According to calculations, the isonicotinic
acid is composed from 4-picoline through the stage of isonicotinic aldehyde formation
and partially decarboxylated at the stage ¹ 3 with pyridine formation. The
presence of 4-cyanopyridine in catalyzates’ experiments indicates that
generated nitrogen-containing fragments at the stage of deep oxidation of initial
4-picoline (stage ¹ 4) and isonicotinic acid (stage ¹ 5) play the role of a nitrogen
donor in relation to aldehyde at the stage ¹ 6 [11].
Experiments for p-nitrotoluene and 3-cyanotoluene
oxidation over V-Ti-catalyst showed that p-nitrobenzonitrile and
isophthalodinitril are formed along with the p-nitrobenzoic acid and m-cyanobenzoic
acid, the formation selectivity of which was 4-10 % per taken starting material
[12]. According to the process scheme (Figure 1), the starting and intermediate
materials are converted towards partial oxidation with aldehydes’ and acids’
formation and towards destructive oxidation, furnishing nitrogen-containing
fragments (potential nitrogen donors) and carbon oxides. Nitrogen donor transfer
from one reaction direction to another in coordination with n-nitrobenaldehyde
and p-nitrobenzoic acid leads to the formation of p-nitrobenzonitrile, which is
an absolute proof of the conjugated process nature. However, the examples of
obvious conjugation are likely special cases. In general terms, conjugation is
more widespread, but usually in an implicit form.
A number of processes based on the coupled
reactions such as oxidative ammonolysis, oxidative dealkylation, obtaining of
terephthalic acid by liquid phase oxidation of p-xylene have been developed in
Kazakhstan under the supervision of academician of NAS RK - B.V. Suvorov.
A number of processes based on the coupled
reactions [13], such as continuous method of producing terephthalic acid by
liquid phase oxidation of p-xylene; process of producing propylene by conjugated
liquid phase oxidation of propylene together with acetaldehyde [14]; conjugated
reactions of hexamethylene dehydrogenation and toluene or o-xylene hydrodealkylation
on palladium membranes [15] have been developed and proposed by B.V. Suvorov
and co-workers.
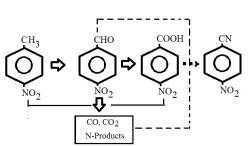
Fig.1. The p-nitrotoluene oxidation process scheme
over V-Ti-catalyst
One of the methods of chemical processes’
formation lies in ammonia substitution in the reaction of catalytical oxidative
ammonolysis for another nitrogen donor and air substitution for another
oxidant. Thus, the process of producing benzonitrile by toluene and ammonia
oxidation of sulfur dioxide on aluminum oxide and other catalysts (the yield of
nitrile is 62%, the selectivity is 92%)
was developed, and called similarly with oxidative ammonolysis
«ammonsulfooxidaton» [16].
The ammonolysis processes have been implemented
into industry for the purpose of producing benzonitrile from toluene, phthalodinitril
from o-xylene, nicotinonitrile from β-picoline, wherein
the movable catalyst oxygen was an oxidizing agent, the reduced form of which was
activated by air in the auxiliary reactor [17]. The process of
«nitrooxydation», wherein benzonitrile is synthesized from Te, Fe, Pb, Sn oxides on the catalyst, with the yield of 67%
by reacting 1 mole of toluene with 2 moles of NO, have been investigested. The
latter acts both as oxidant, and nitrogen donor [18].
Based on analysis of literature data array on
catalytic oxidation reactions we made a conclusion that it is possible to
isolate group reactions, which on its phenomenological characteristics approach
to the reaction of oxidative ammonolysis mentioned above. They all take place
by a single scheme: the starting material + coreactant + oxidizing agent + catalyst
→ reaction product (usually not containing oxidizing agent in its
compound). Examples of such reactions are shown in Table 2. A lot of them have analogues
carried out in the liquid phase, for example, the catalytic and non-catalytic
oxidative ammonolysis [11], oxidative chlorination, oxidative etherification [11].
Of course, such different reactions do not
proceed by the same mechanism. However, common phenomenological characteristics
(performing reactions in the presence of oxidizing agents and as a rule,
semiconductor catalysts, dependence of speed and processes’ selectivity on
additions of various substances in the starting reaction mixture, producing the
desired product which does not have oxidizing agents in its compound) were used
as a reason to define the generality in the regularities of these processes
[11, 19, 20].
Table 2 - Complex
gas-phase oxidation processes.
|
Starting
materials |
Catalyst, temperature |
Basic
process product |
References |
|
|
1.
Oxidative alkylation |
||||
|
Benzene, ethylene, Î2 |
Pt, 2000C |
Styrole Ñ6Í5ÑÍ=ÑÍ2 |
11, p. 97 |
|
|
Benzene, methane, Î2 |
FeOX,TiOX, 6000C |
Ethylbenzene + Styrole |
||
|
2.Oxidative
hydroxylation |
||||
|
Benzene methanol, Î2 |
Silica, Â2Î3,
6400C |
Phenol |
22 |
|
|
3.
Oxidative chlorination |
||||
|
Ethylene, 2HCI, 0,5 Î2 |
CuCl2 ,2000C |
Dichloroethane |
11, ch.8 |
|
|
Benzene, HCI, 0,5 Î2 |
Cu, Fe chlorides, 2150C |
|||
|
4.
Oxidative
etherification |
||||
|
Ethylene, ÑH3COOH, Î2 |
PdCl2 , 160-2000C |
Vinyl acetate CH2=ÑÍ-OÑO-CH3 |
11,ch.5 |
|
|
Ñ6Í5ÑOOÍ, ÑÍ3OÍ, Î2 |
VXOY |
Ñ6Í5ÑOOCH3 |
||
|
5.
Oxidative cyanation |
||||
|
Methane, acetonitrile,
Î2 |
Silica,
6000C |
CH3ÑÍ2ÑN + CH2=ÑÍÑN |
11, ch.4 |
|
|
Styrole, HCN, Î2 |
PdCl2, KCl, 2500C |
Ñ6Í5ÑÍ=ÑÍCN |
||
|
6.
Oxidative heterocyclization |
||||
|
CH2=ÑÍÑÍÎ, NH3, Î2 |
Cu, Zn, Pb, Ag oxides, 350-4000C |
Pyridine, 3-methylpyridine (β-picoline) |
11, p. 99 |
|
|
7.
Oxidative ammonolysis |
||||
|
CH2=ÑÍÑN, NH3, Î2 |
Bi, Mo oxides, 250-4500C |
CH2=ÑÍÑN, acrylonitrile |
11, ch. 6, 23 |
|
|
Substituted alkylbenzenes (substitutes – haloids, oxy-, alkoxy-, nitro
group), NH3 , Î2 |
V,Ti, Mo, B, P oxides, 350-4000C |
Benzonitrile, di-, tri-cyanbenzenes and corresponding derivatives with
haloids, oxy-, alkoxy-, nitro groups |
||
|
Pyridine, 2-, 3- è 4- methylpyridines |
V,Ti, Mo, Bi oxides, 350-4000C |
2-, 3-, 4- cyanpyridines |
||
|
8.
Ammonsulfooxidation |
||||
|
Toluene, NH3 ,SÎ2 |
Al, Si, Zr, V oxides, 4000C |
Benzonitrile |
11, p. 136 |
|
|
9.
«Lammas»-process |
||||
|
Substituted alkylbenzenes,
NH3 |
V superoxides, 4000C |
Benzonitrile and
corresponding derivatives |
11, p. 132,155-157 |
|
|
10. Oxidative nitrilling |
||||
|
Toluene, HCN or CH3CN, Î2 |
Cu,V,Ti
oxides, 400-4500C |
Benzonitrile |
11, p. 110, 422 |
|
|
Toluene, nitrobenzene or aniline, Î2 |
V,Ti
oxides, 400-6000C |
|||
It can be concluded that a
significant number of complex reactions mentioned above are genetically connected
with heterogeneous oxidation reactions conducted in various extreme conditions.
Also reactions, in which the main direction of the process is caused by
non-oxidative conversions, can be associated, for example, the reaction of
oxidative etherification. However, here too
the oxidizing agent is required for the total process. In most considered
reactions the catalyst operates according to reductive-oxidative mechanism, where
the oxidizing agent performs the function of reoxidation of reductive active
catalyst sites by organic matter and co-reactant. The conjugated process
character have been proven for a number of processes such as oxidative
ammonolysis, ammonsulfooxidation, conjugated oxidative dehydrogenation,
oxidative chlorination [11]. However, the conjugation role is still an open
question, therefore, all processes shown in the table should be primarily
attributed to a complex combined processes including stages of different
consecutive, parallel and coupled reactions.
The method of chemical
processes’ formation by introducing an oxidizing agent into a mixture of basic
co-reactants, the components of which are part of the desired product, was
quite productive. It allowed to create such leading industrial processes as
oxidative chlorination, oxidative ammonolysis, oxidative etherification, according to which modern bulk
products - vinyl chloride, acrylonitrile and vinyl acetate have been produced
[21]. Such well-known polymeric materials as polyvinyl chloride (manufacture of
double-glazing windows, linoleum, packaging materials), polyacrylonitrile
(manufacture of "nitrone" fiber, carbon fiber-reinforced plastic),
polyvinyl acetate (PVA building adhesive, the starting material for the
polyvinyl alcohol used to prepare the high modulus fibers and others) are
obtained from these monomers. Other examples of complex reactions are given in works
[22, 23].
REFERENCES
1. B.V. Suvorov, S.R. Rafikov, A.D. Kagarlitskiy. The oxidative
ammonolysis of organic compounds // Usp. - 1965. - T.35, ¹9. - p. 1526-1549;
2. B.V. Suvorov. The oxidative ammonolysis of organic compounds. -
Almaty, Science of the Kazakh SSR, 1971. – p. 146;
3. B.V. Suvorov in the book; V.A. Volkov, E.V. Vonskiy, G.I. Kuznetsova.
Outstanding chemists of the world. Edited by G.I. Kuznetsova - M.: High School,
1991. – p. 656;
4. N.N. Shilov. Conjugate oxidation reactions. - M., 1905. - p.304;
5. N.M. Emanuel, D.G. Knorre. Course of chemical kinetics, 4th ed. - M.,
High School, 1984. - p. 463;
6. N.R. Bukeikhanov, B.V. Suvorov, B.T. Dzhusupov and others. The
conjugated character of contact oxidation of some nitrogen-containing organic
compounds // Math. Academy of Sciences of the Kazakh SSR, Ser. chem. - 1977,
¹3. - p.48-52;
7. P.B. Vorobyev, T.P. Mikhailovskaya, R. Kurmakyzy, A.B. Dikhanbayev, D.H.
Sembaev. Partial oxidation of 4-picoline on V2O5 and
promoted vanadium oxide catalysts // Chem. Journal. of Kazakhstan. - 2010, ¹ 4.
- p. 58-66;
8. L.S. Polak, M.J. Goldenberg, A.A. Levitskyi. Computational methods in
chemical kinetics. - M., 1984. - p. 280;
9. D. Himmelblau. Experimental nonlinear programming. - M., 1975. - p.536;
10. V.F. Kiselev, O.V. Krylov. Electronic effects in adsorption and
catalysis on semiconductors and dielectrics. - M., 1979. - p.236;
11. B.V. Suvorov, N.R. Bukeikhanov. Oxidation reactions in organic
synthesis. - M. Chemistry, 1978. - p.197;
12. B.T. Dzhusupov, N.R. Bukeikhanov, B.V. Suvorov. Synthesis of
aromatic nitriles by oxidation of alkylbenzenes with organic nitrogen-containing
compounds // Math. Academy of Sciences of the Kazakh SSR, Ser. chem. - 1977, ¹
4. - p. 66-70;
13. L.G. Manukovskaya, A.V. Solomin, B.V. Suvorov, S.R. Rafikov.
Continuous method of producing terephthalic acid by liquid phase oxidation of
p-xylene // Petrochemistry. - 1962. - B. 2, ¹4. - p. 531-535;
14. N.M. Emanuel, O.N. Dyment, E.A. Blumberg. Modern methods of ethylene
oxide and propylene oxide production // Journal. WMO named after D.I.
Mendeleev. - 1969. - B.14, ¹3. - p. 238-262;
15. V.M. Gryaznov. Reaction conjugation by using membrane catalysts // Kinetics
and catalysis. -1971. - B.12, issue 3. - p. 640-645;
16. H. Hoffmann. Ammonsulfooxidation
// Chem. Zeit., Chem.Apparat. - 1967, ¹12. - P. 392-398;
17. J.E. Paustian,
F. Pustio, N. Stavropouls, M. Sce. A lesson inflow sheet design nicotinamide
and acid // Chem.Techn. –1981. – V.11, ¹ 3. – p. 174-178;
18. E. Fisher.
Preparation of aromatic nitriles by means of a new vapor phase reaction //
Chem.Ind.Techn. – 1966. – Bd.38. – p. 35-37;
19. V.S. Zulkasheva, N.R. Bukeikhanov, B.V. Suvorov. The oxidative
ammonolysis of mixtures of some methylbenzenes // Iss. Academy of Sciences of
the Kazakh SSR, Ser. chem. - 1980, ¹5. – p. 48-51;
20. B.V. Suvorov, N.R. Bukeikhanov, L.V. Li. Ways of processes intensification
of nitriles production on the basis of oxidative ammonolysis reaction of
organic substances // In book.: Synthesis and study of monomers and polymers. -
Alma-Ata, Science. - 1983. - p. 3-23;
21. L. Berdick Donald, William L. Leffler Petrochemistry/ trans. from
English. - M .: CJSC "Olymp-Business." - 2001. - p. 416;
22. D.R. Ashmead Brit. Pat. ¹
1274653, 1972. Oxydation of Hydrocarbons.
23. N.R. Bukeikhanov, B.V. Suvorov. Synthesis of substituted aromatic
nitriles. - In coll.: Chemistry of monomers and polymers. - Alma-Ata, Science,
1980. - p. 3-15.