ISOTOPIC COMPOSITION OF WATER AND ITS TEMPERATURE IN MODELLING PRIMODIAL
HYDROSPHERE EXPERIMENTS
1 Scientific
Research Center of Medical Biophysics (SRCMB), 1111, Sofia, N. Kopernik, 6,
Bulgaria, e-mail: mbioph@dir.bg
2 Moscow State university of applied biotechnology,
109316, Moscow, Talalikhina, 33,Russia,
e-mail: mosin-oleg@yandex.ru
Natural prevalence of deuterium
makes up approximately 0,015 at. % D, and
depends strongly on the uniformity of substance and the total amount of matter
formed in the course of early evolution [1]. Constant sources of deuterium are explosions of nova stars and
thermonuclear processes occurring inside the stars. Probably, it could explain
a well known fact why the amounts of
deuterium are increased slightly during the global changes of climate in
worming conditions. Gravitational field of the Earth is
insufficiently strong for retaining of lighter hydrogen, and our planet is
gradually losing hydrogen as a result of its dissociation into interplanetary
space. Hydrogen evaporates faster than heavy deuterium which is capable to be
collected by the hydrosphere. Therefore, as a result of this natural process of
fractionation of isotopes H/D throughout the process of Earth evolution there
should be an accumulation of deuterium in hydrosphere and surface waters, while
in atmosphere and in water vapor deuterium contents are lower. Thus, on the
planet there is going on a natural process of separation of H and D isotopes,
playing an essential role in maintenance of life on the planet.
The absolute
contents of deuterium (isotopic shifts, δ, ppm) according to the
international standard VSMOW, corresponding to Pacific ocean water which is
rather stable on isotopic composition, compile D/H = (155,76±0,05).10-6
(155,76 ppm) [2]. For the international standard SLAP of natural water of
Antarctic Region containing less deuterium, the absolute contents of deuterium
compile D/H = 89.10-6 (89 ppm). The average ratio of H/D
in nature compiles 1 : 5700. In natural waters the contents of deuterium are
distributed non-uniformly: from 0,015 at.% D for water from the Antarctic ice -
the most deuterium depleted natural water with deuterium contents in 1,5 times
smaller, than in sea water, up to 0,02-0,03 at.% D for river and sea water.
Thawed snow and glacial waters in mountains and some other regions of the Earth
usually contain on 3-5% less deuterium, than drinking water. On the average, 1
ton of river water contains approximately 150-300 g of deuterium. Other natural
waters contain varying levels of deuterium from δ = +5,0 D, %, SMOW
(Mediterranean Sea) up to δ = -105 D, %, SMOW (Volga River).
The peposition
was made by us that primary water could contain more deuterium in early
stages of evolution, and deuterium was distributed non-uniformly in hydrosphere
and atmosphere [3]. As is known, the primary reductive atmosphere of the Earth,
consisted basically of gas mixture CO, H2, N2, NH3,
CH4, was lacked O2–O3 layer protecting the
Earth surface from rigid short-wave solar radiation carrying huge energy capable to cause photolysis and
radiolysis of water. The processes accompanying accumulation of deuterium in hydrosphere were solar radiation, volcanic geothermal
processes and electric categories in electric discharges in atmosphere. These natural processes could lead to enrichment of hydrosphere by deuterium in the form of HDO which evaporates more slowly
then H2O, and condenses faster. The formation of HDO occurs in D2O-H2O
mixtures via isotopic exchange: Í2O + D2O = 2ÍDO, causing
deuterium at small amounts to be present in water in form of ÍDO, and at high
amounts - in form of D2O. The structure of molecules D2O is the same, as that of Í2O, with very
small distinction in values of lengths of covalent bonds. D2O boils at 101,44 0Ñ,
freezes at 3,82 0Ñ, has density at 20 0Ñ 1,105 ã/sm3,
and the maximum of density is not on 4 0Ñ, as for usual water, but
on 11,2 0Ñ (1,106 ã/sm3). These effects are reflected in energy
of a chemical bond, kinetics and
chemical reactions rates in D2O-H2O
mixtures. Enzymic reactions in D2O are considerably slowed down. However, there are also
such reactions which rates in D2O are higher, than in Í2O.
Basically, they are reactions catalyzing by ions D3Î+ or
H3Î+ or OD- and OH-. According to
the theory of chemical bond, breaking up of H-O bonds can occur faster, than
D-O bonds, mobility of ion D3O+ is lower on 28,5 % than Í3O-
ion, and ÎD- ion is lower on 39,8 % than OH- ion,
the constant of ionization of D2O is less than constant of
ionization of H2O [4]. The maximum kinetic isotopic effect at ordinary temperatures in a chemical reaction
leading to rupture of bonds involving H and D was calculated, and the maximum
ratio kh/kd in macromolecules is in the range of 6 to 8 for C-H
versus C-D, N-H versus N-D, and O-H versus O-D bonds [5].
Deuterated cells of various
microorganisms adapted to the maximal concentration of D2O in growth
media (95-98 vol.% D) are convenient objects for evolutional and adaptation
studies as well as structural-functional studies. During the cellular growth on
D2O media there are synthesized macromolecules in which hydrogen
atoms in carbon skeletons are almost completely replaced on deuterium. Such
deuterated macromolecules undergo the structural-adaptive modificational
changes necessary for normal functioning of cells in the presence of D2O.
Practical interest to further
applying of deuterated cells of various microorganisms in researches on their
basis mechanisms of cellular adaptation to D2O and molecular
evolution, has predetermined a direction of our studies. The purpose of the present
reseach was studying of isotope effects of deuterium and conditions of primary
hydrosphere (temperature, value ðÍ, isotopic composition).
In frames of the research were studied various samples of water from
Bulgaria.
We have investigated isotopic effects
of deuterium in prokaryotic and eukaryotic cells of various taxonomic groups of
microorganisms realizing methylotrophic, hemoheterotrophic, photoorganotrophic
and photosynthetic ways of assimilation of carbon substrates (methylotrophic
bacteria, halobacteria, blue-green algae) in D2O with using 1H-NNR-,
IR-, and mass-spectrometry technique. The method of step by step adaptation is
developed for adaptation of cells of various microorganisms to D2O
consisting in plating initial cells on firm (2% agarose) growth media with
increasing gradient of D2O concentration (from 0; 24,5; 49,0; 73,5
to 98 % D2O) and the subsequent selection of clones
resistent to deuterium. Cells grown on media with a low gradient of D2Î concentration were transferred on media with big gradient of
concentration, up to 98 % D2Î. Degree
of cell survive on maximum deuterated media was about 40%.
Our experiments demonstrated, that
the effects observed at the cellular growth on D2O possess complex
multifactorial character connected to changes of morphological, cytologic and
physiological parameters – magnitude of the log-period, time of cellular
generation, outputs of biomass, a ratio of amino acids, protein, carbohydrates
and lipids synthesized in D2O, and with an evolutionary level of organization
of investigated object as well. The general feature of bacterial growth in D2Î was the proportional increase in duration of the log-period and time of
cellular generation at reduction of outpunts of a microbic biomass. The
experimental data testify that cells realize the special adaptive mechanisms
promoting functional reorganization of work of the vital systems in the
presence of D2O. Thus, the most sensitive to replacement of Í+ on D+ are the apparatus of biosynthesis of
macromolecules and a respiratory chain, i.e., those cellular systems using high
mobility of protons and high speed of breaking up of hydrogen bonds. Last fact
allows consider adaptation to D2O as adaptation to the nonspecific
factor effecting simultaneously functional condition of several numbers of
cellular systems: metabolism, ways of assimilation of carbon substrates,
biosynthetic processes, and transport function, structure and functions of
macromolecules. There is evidence that during adaptation to D2O the
ration of synthesized metabolites is changing. Furthermore, deuterium induces physiological, morphological and cytological
alterations in the cell. This leads to the formation in D2O
of large atypical
cells [6, 7]. They are
usually 2–3 times larger in size and have a thicker cellular
wall compared to the control cells grown on H2O. The structure of DNA in deuterated
cells in D2O may alters; distribution of DNA in them was non-uniform. The data obtained confirm
that adaptation to D2O is a phenotypical
phenomenon as the adapted cells return back to normal growth after some
log–period after their replacement into H2O. At the same time the
effect of convertibility of growth on H2O/D2O does not
exclude an opportunity that a certain genotype determines displaying of the
same phenotypical attribute in D2O.
Experiments with D2O
have shown (fig. 1), that green-blue algae is capable to grow on 70% D2O,
methylotrophic bacteria – 75% D2O, chemoheterotrophic bacteria – 82%
D2O, and photoorganoheterotrophic bacteria – 95 % D2O.
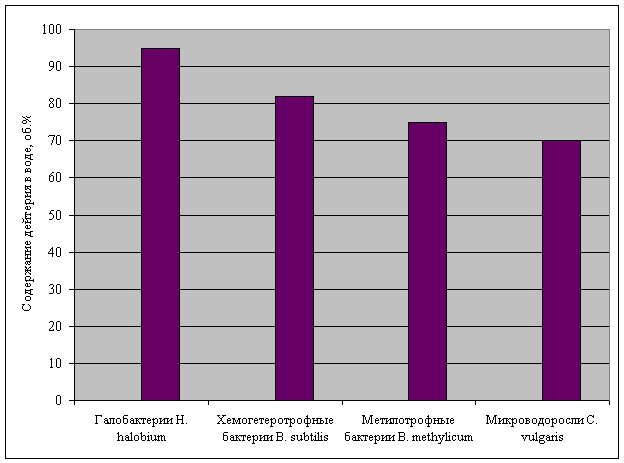
Fig. 1. Survival rate of cells of the
studied microorganisms in water with various content of deuterium.
In the process of adaptation to D2O
the most important for macromolecular structure are dynamic ahort-lived
hydrogen (deuterium) bonds formed between the neighbor atoms of H(D) and O, C,
N, S- heteroatoms, playing an essential role in maintenance of spatial
structure of macromolecules and intermolecular interactions. The substitution of H with D atom affects the
stability and geometry of hydrogen bonds in apparently rather complex way and
may, through the changes in the hydrogen bond zero-point vibrational energies,
alter the conformational dynamics of hydrogen (deuterium)-bonded structures of
DNA and protein in D2O. It may cause disturbances in the
DNA-synthesis, leading to permanent changes in DNA structure and consequently
in cell genotype. The multiplication which would occur in macromolecules of
even a small difference between a proton and a deuteron bond would certainly
have the effect upon the structure. The sensitivity of enzyme function to the
structure and the sensitivity of nucleic acid function (genetic and mitotic)
would lead to a noticeable effect on the metabolic pathways and reproductive
behavior of an organism in the presence of D2O. And next, the
changes in dissociation constants of DNA and protein ionizable groups when
transferring the macromolecule from H2O to D2O may
perturb the charge state of the DNA and protein molecules. Other
important property is defined by the
three-dimensional structure of D2O molecule having
the tendency to pull together hydrophobic
groups of macromolecules to minimize their disruptive effect on the hydrogen
(deuterium)-bonded network in D2O. This leads to stabilization of the
structure of protein and nucleic acid macromolecules in the presence of D2O
[8]. At placing a cell in D2O, not only H2O is removed
from a cell due to reaction of D2O dissociation, but also there is
occurred fast isotopic (H–D) exchange in hydroxyl (-OH), sulfhydryl (-SH) and amino groups (-NH2)
of all organic substances, including proteins, nucleic acids, carbohydrates and
lipids. It is known, that in these conditions only covalent C-H bond is not
exposed to isotopic (H-D) exchange and, thereof only substances with bonds such
as C-D can be synthesized de novo [9].
Biological experiments with D2O and structural-conformational
studies enable to modelling conditions under which life has evolved. The most
favorable are accepted alkaline mineral waters interacting with CaCO3
and then sea waters [10]. Circulating in bowels on cracks, crevices, channels
and caves karst waters are enriched with Ca(HCO3)2,
actively cooperating with live matter. Once appeared in these waters the
process of self-organization of primary organic forms in water solutions may be
supported by thermal energy of magma, volcanic activity and solar radiation.
In connection
with these data are important the following reactions:
(1) CO2 + 4H2S
+ O2 = CH2O + 4S + 3H2O
(2) ÑàÑÎ3+ HOH + ÑÎ2
= Ca(HCÎ3)2
(3) CO2
+ ÎÍ- = HCÎ3-
(4) 2 HCO3-
+ Ca2+ = CaCO3 + CO2 + H2O
The equation
(1) shows how some chemosynthetic bacteria use energy from the oxidation of H2S
to S. The equation (2) is related to formation of Ca(HCÎ3)2 from H2O, ÑÎ2 and ÑàÑÎ3. In the presence of
hydroxyl OH- ions ÑÎ2 transforms into HCÎ3- (equation (3). Equation (4) is valid for the process
of dolomite formation of stromatolites.
Furthermore, we have carried out
the research of mineral, sea and mountain water from Bulgaria by
IR-spectroscopy method of differential non-equilibrium energy spectrum (DNES)
relative to the control – deionized water (fig. 2, curves 1-5, the
table). In experiments were investigated samples of water from karst springs.
Also IR-spectra of castus juice were investigated by DNES method (fig. 2, curve
1). The cactus was selected as a model system because the plant contains
about 90% water. The closest to the IR-spectrum of castus juice was the
IR-spectrum of the mineral water contacting with ÑàÑÎ3 (fig. 2, curve 2). IR-spectra of plant juice, mineral water and
water of the kars springs have magnitudes of peaks in IR-spectra at -0,1112; -0,1187; -0,1262; -0,1287 and -0,1387 eV,
accordingly. Similar peaks in the IR-spectrum between cactus juice, mountain
and sea water were detected at -0,1362 eV. The IR-spectrum of the control
sample of deionized water (fig. 2, curve 5) was substantially different
from the IR-spectrum of sea mineral and mountain water. The values of average
energy (∆EH... O) of hydrogen Í…O-bonds between
molecules H2O in the process of formation of (H2O)n associates,
measured by the DNES method were measured at 0,1067±0,0011 eV.
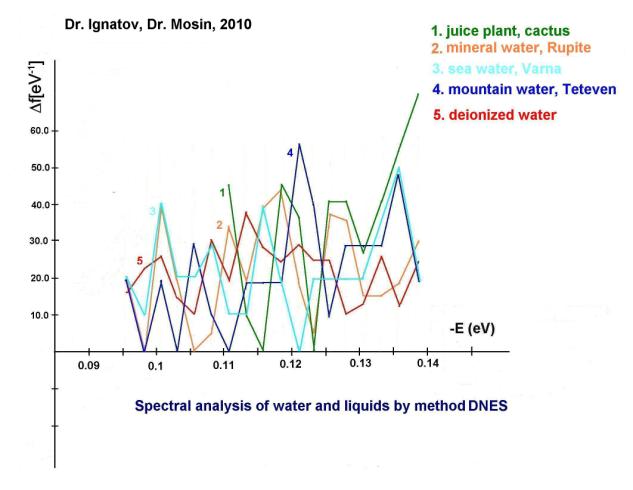
Fig. 2. ÄNES spectra of water of various origin: 1 –
cactus juice; 2 – mineral water Rupite (Bulgaria); 3 – sea water (Varna, Bulgaria); 4 – mountain water (Teteven, Bulgaria); 5 –
deionized
water (control).
The table. Characteristics of IR-spectra of water of various origin obtained by
DNES-method.
|
-Ex (eV) Cactus juice |
-E (eV) Mineral water Rupite |
-E (eV) Sea water |
µm |
cm-1 |
|
0,1112 |
0,1112 |
|
11,15 |
897 |
|
0,1187 |
0,1187 |
|
10,45 |
957 |
|
0,1262 |
0,1262 |
|
9,83 |
1017 |
|
0,1287 |
0,1287 |
|
9,64 |
1037 |
|
0.1362 |
|
0,1362 |
9,10 |
1099 |
|
0,1387 |
0,1387 |
|
8,95 |
1117 |
The data obtained proved
that hot mineral alkaline water is preferable for maintanence of life. These
data also can predict a possible way of transition from synthesis of small
organic molecules due to the energy of UV solar radiation and thermal activity
to more complex organic molecules as protein and nucleic acids. The important
factor in reaction of condensation of two molecules of amino acids is
allocation of H2O molecule when peptide chain is formed. As reaction
of polycondensation of amino acids is accompanied by dehydratation, the H2O
removal from reactional mixture speeds up the reaction rates. This testifies
that formation of organic forms may occur nearby active volcanoes, because at
early periods of geological history volcanic activity occurred more actively
than during subsequent geological times. However, dehydratation accompanies not only amino acid polymerization,
but also association of other blocks into larger organic molecules, and also
polymerization of nucleotides into nucleic acids. Such association is connected
with the reaction of condensation, at which from one block removes proton Í+, and from another – hydroxyl group
(OH-) with formation of H2O molecule.
The possibility of
existence of condensation-dehydratation reactions under conditions of primary hydrosphere
was proven by Calvin in 1965 [11]. From most chemical substances hydrocyanic
acid (HCN) and its derivatives – cyanoamid (HNCN2) and dicyanoamid
(HN(CN)2) possess dehydratation ability and the ability to catalyze the process
of linkage of H2O from primary hydrosphere [12]. The presence of HCN
in primary hydrosphere was proven by Miller's early experiments. Chemical
reactions with HCN and its derivatives are complex with chemical point of view;
in the presence of HCN, HNCN2 and HN(CN)2 the condensation of separate blocks of amino
acids accompanied by dehydratation, can proceed at normal temperatures in
strongly diluted H2O-solutions. Furthermore, polycondensation of
amino acids in the presence of HCN and its derivatives depends on acidity of
water solutions in which they proceed [13]. In acid water solutions (ðÍ 4–6) these reactions do
not occur, whereas alkaline conditions (ðÍ 8–9) promote their course.
In synthesis of organic
molecules other energy sources, e. g. geothermal sources could be used. In 2011
a team of Japanese scientists led by T. Sugawara created a membrane like proto
cells from aqueous solution of organic molecules, DNA and synthetic enzymes
under temperature close to water’s
boiling point 950Ñ [14]. These laboratory
experiments is an excellent confirmation of the possibility that life
originated in hot water.
The data obtained
testify that life
maintanence
depends on phisical-chemical properties of water and external factors – temperature, ðÍ. Hot mineral alcaline water, which
interacts with CaCO3 is closest to these conditions. Next in line
with regard to quality is sea and mountain water. In warm and hot mineral waters IR-peaks in DNES spectra were more expressed in comparison with the
IR-peaks received in the same
water with lower temperature. The spectral range of DNES was in the middle infrared
range from 8 to 14 mm. It is thought that
there is the Earth atmosphere’s window of transparency for the electromagnetic
radiation in the close and middle infrared range. In this interval energy is
radiated from the Sun towards the Earth, and from the Earth towards surrounding
space. If in the primodial hydrosphere was much more deuterium, this is a
significant fact regarding thermal stability of deuterated macromolecules in
the preservation of life under thermal conditions.
References
1. Linsky, J.L. D/H and nearby interstellar cloud structures, Space
Science Reviews, NY: Springer Science, Business Media, 2007, V. 130, p. 367; Linsky, J.L. et al.
What is the total deuterium abundance in the local Galactic disk? //
Astrophysical Journal, 2007, V. 647, p. 1106.
2. Lis G., Wassenaar L.I., Hendry M.J. High-Precision
Laser Spectroscopy D/H and 18O/16O Measurements of
Microliter Natural Water Samples // Anal. Chem., 2008, V 80 (1), p. 287-293.
3. Mosin O. V. Deuterium, heavy water, evolution and
life // Vodoochistka, vodopodgotovka, vodosnabzhenije, 2009. ¹ 8, p. 64-70.
4. Lobishev V. N., Kalinichenko L. P. Isotopic effects
of D2O in biological systems M.: Nauka, 1978, 215 p.
5. Vertes A. Physiological effects of
heavy water. Elements and isotopes: formation, transformation, distribution. - Dordrecht:
Kluwer Acad. Publ., 2004, 112 p.
6. Mosin O. V., Skladnev D. A., Shvets V. I. Studying
of physiological adaptation to heavy water // Biotechnologija, 1999. ¹ 8, p.
16-23.
7. Mosin O. V., Skladnev D. A., Shvets V. I. Methods
for production of proteins and amino acids, labelled with stable isotopes 2Í, 13Ñ è 15N // Biotechnologija, 1996. ¹ 3, p. 12-32.
8. Mosin O. V., Ignatov I. Isotopic effects of
deuterium in cells of bacteria and microalgae // Water: chemistry and ecology,
2012. ¹ 3, p. 83-94.
9. Ignatov, I., Energy Biomedicine, Origin of Living
Matter, “Informationability of water, Bioresonance, Biophysical Fields,
Institute for Creative Healing, Munich (2007).
10. Ignatov, I., Which water is optimal for the origin
(generation) of life? EUROMEDICA, Hanover, (2010).
12. Mathews C.N., Moser R. Peptide synthesis from
hydrogen-cyanide and water // Nature, 1968, V. 215, p. 1230-1234.
13. Abelson P. Chemical events on the"primitive
earth. // Proc. Natl. Acad. Sci. U. S., 1966, V. 55, p. 1365-1372.
14. Ò.
Sugawara. “Self-reproduction of supramolecular giant vesicles combined with the amplification of encapsulated DNA”, Nature
Chemistry, 2011. V. 1127, p.
775-780.